
The gating process of VDAC is inhibited by trace amounts of aluminum in solution. This was first observed when the use of disposable syringe needles, constructed with aluminum hubs, was linked to poor gating of VDAC channels. The detective work of Tracy Dill resulted in identifying aluminum as the culprit. Surprisingly it is not the trivalent aluminum (Al3+) that is responsible for the phenomenon but rather the neutral aluminum hydroxide (Al(H2O)3(OH)3) . This is the species most prevalent at neutral pH. However this is also the species that forms an insoluble precipitate. It seems that aluminum hydroxide exists, for long periods of time, in solution, out of equilibrium, in a metastable state.
That this is the species that reduces the voltage dependence of VDAC was shown in a variety of ways. First of all simply reducing the pH, which increases the concentration of trivalent aluminum ions, reverses the inhibition (Dill et al., 1987). However, the most convincing evidence comes from the thorough work of Dawei Zhang (1989). He examined aluminum and other ions as a function of pH. Not surprisingly, other elements in the same group in the periodic table as aluminum, gallium and indium, also inhibit the voltage-gating of VDAC but the potency of their inhibition depends on the amount of the trihydroxide that is present under the conditions used, mainly as a function of pH. Indium is the most potent because it is able to produce proportionally higher amounts of the indium hydroxide as a species in solution. Other effective ions are scandium, chromium, and trivalent iron. All these form trihydroxide ions. Chromium is of particular interest because its trihydroxide tends to precipitate rather readily and, as it does so, the effectiveness of the inhibition declines.
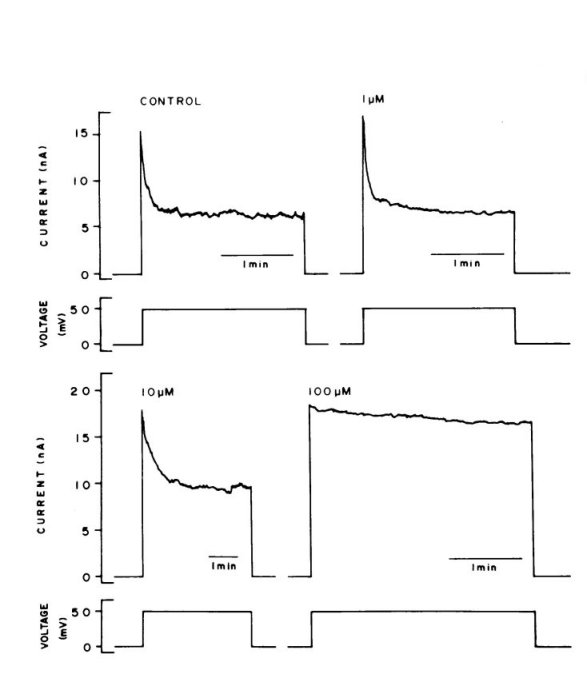
The potency of these cations is evident. Effects are easily seen with
10 mM aluminum (see figure) and sub-micromolar
indium. No effects were observed with divalent metal hydroxide such as
those formed by zinc and cobalt and no effect was observed with trivalent
lanthanum.
| |
dependence |
to cause inhibition |
|
| |
|||
| chloride | |
|
|
| citrate | |
|
|
| malate | |
|
|
| pyruvate | |
|
|
| borate | |
|
|
| |
|||
| Li (I) | |
|
|
| Na (I) | |
|
|
| Mg (II) | |
|
|
| Al (III) | |
|
|
| K (I) | |
|
|
| Ca (II) | |
|
|
| Sc (III) | |
|
|
| Cr (III) | |
|
|
| Fe (III) | |
|
|
| Co (II) | |
|
|
| Cu (II) | |
|
|
| Zn (II) | |
|
|
| Ga (III) | |
|
|
| In (III) | |
|
|
| La (III) | |
|
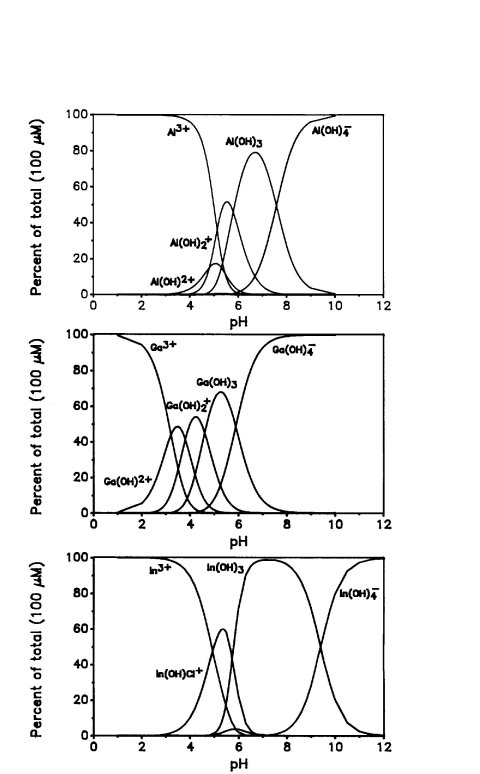
The effects of these metals on the properties of VDAC are consistent with an effective neutralization of the VDAC's voltage sensor. However inhibition by aluminum does not alter the selectivity of the channels indicating that there is no real neutralization of charge that is facing the ion stream. Thus either aluminum hydroxide is binding and neutralizing charge on the surface domains or the binding of aluminum hydroxide somehow reduces the steepness of the voltage dependence without neutralizing the charge on the sensor. This puzzling observation misled us in our thinking about and our probing the structure and location of the voltage sensor. It is still unclear how this fits into the overall process. At the time VDAC was thought to be a dimer and binding sites for aluminum on the two sides of the membrane made sense. Now that we know that VDAC is a monomer, the dual effect of aluminum from either side, and the asymmetry of the effect of aluminum when it is added to one side only, is rather puzzling.
The role of VDAC in aluminum toxicity is an unexplored area.
References:
Dill, E. T., Holden, M. J. and Colombini, M. 1987. Voltage gating in VDAC is markedly inhibited by micromolar quantities of aluminum. Journal of Membrane Biology, 99: 187-196.
Zhang, D. W. and Colombini, M. 1989. Inhibition by aluminum hydroxide of the voltage-dependent closure of the mitochondrial channel, VDAC. Biochimica et Biophysica Acta, 991: 68-78.
Zhang, D. W. and Colombini, M. 1990. Group IIIA-Metal hydroxides indirectly neutralize the voltage sensor of the voltage-dependent mitochondrial channel, VDAC, by interacting with a dynamic binding site. Biochimica et Biophysica Acta, 1025: 127-134.
Ahmadzadeh, M., Horng, A., and Colombini, M. 1996. The Control of Mitochondrial Respiration in Yeast: A Possible Role of the Outer Mitochondrial Membrane. Cell Biochem. Funct., 14: 201-208. (aluminum was used to attempt to control the state of the channels)
Please
send comments and contributions to Dr.
Marco Colombini |
Last modified: March 27, 2004 15:24 Page created and maintained by Swapnil Sharma |