
 |
Research Projects
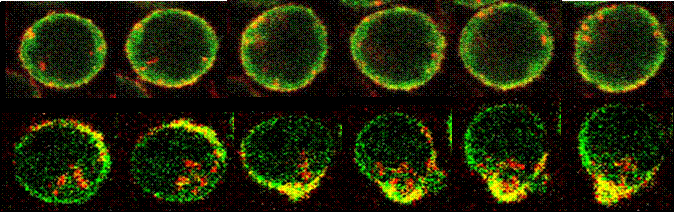
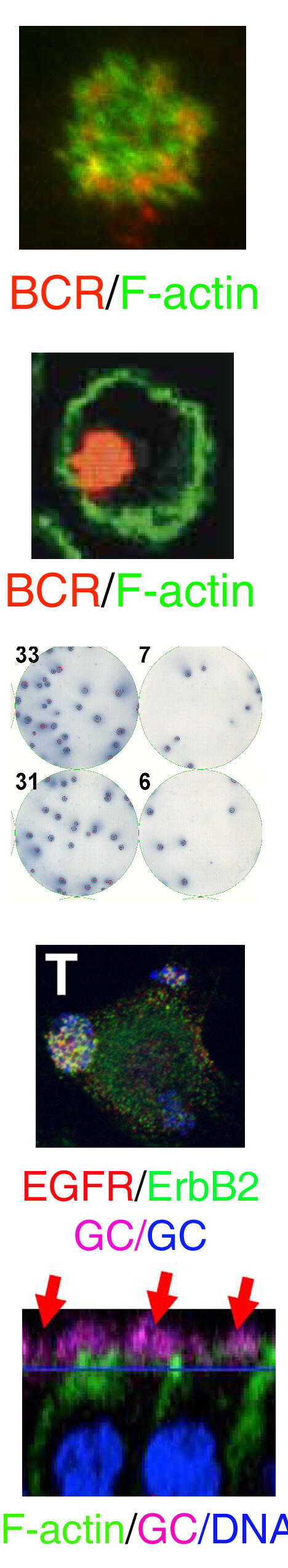
Crosstalk between the actin cytoskeleton and the signaling pathways of the B cell receptor (BCR): B cell activation is initiated by the binding of antigen to the BCR. The actin cytoskeleton plays an essential role in BCR activation. Our recent study shows that both positive and negative signals can regulate the actin remodeling, and the actin reorganization in turn modulates the self aggregation and lateral movement of surface BCRs and B cell spreading on antigen tethered membranes, providing feedback to signal transduction. This project examines the molecular mechanism by which the actin cytoskeleton interplays with the signaling pathways of the BCR.
The actin cytoskeleton in BCR-mediated antigen processing and presentation: The specificity of the BCR to antigen and BCR-induced signaling significantly increase the efficiency of B cells to processing and present antigen. Our previous studies show that BCR signaling activation increases BCR internalization and targeting to the antigen processing compartment, and that BCR internalization requires BCR-induced actin remodeling. We are interested in the cellular mechanism for the transition of surface BCRs from the signaling to endocytosis mode on the cell surface. We are examining how the actin cytoskeleton coordinates BCR signaling to facilitate this transition.
The cell biology of memory B cells: Memory B cells are responsible for maintaining the level of protective antibodies and generating robust antibody responses when re-encountering a previously exposed antigen. We previously showed that specific antigen, but not non-antigenic stimulation (such as TLR agonists), is essential for the activation of memory B cells in vivo. We are interested in the cellular mechanism by which memory B cells are maintained and activated.
The interaction of Neisseria gonorrhoeae with polarized genital epithelial cells: N. gonorrhoeae initiates infection in the female reproductive tract by interacting with cervical epithelial cells. Our recent studies show that N. gonorrhoeae can induce the activation of epidermal growth factor receptor (EGFR) by increasing the expression and surface cleavage of EGFR ligands, and the EGFR activation is important for bacterial invasion. Currently, we are investigating how gonococci utilize host signaling to facilitate their adherence to, invasion into, and transmigrate across the epithelial barrier lining along the female reproductive tract.
The impact of female sex hormones on the interaction of gonococci with epithelial cells: The function and organization of the epithelium in the female reproductive tract are regulated by sex steroid hormones that control the menstrual cycle. Gonococcal symptomatic infections in women are positively associated with the first week of the menstrual cycle. We are interested in understanding how gonococci adapt into the unique hormonal changes, establish infections, and cause diseases in the female reproductive tract.