




 | |

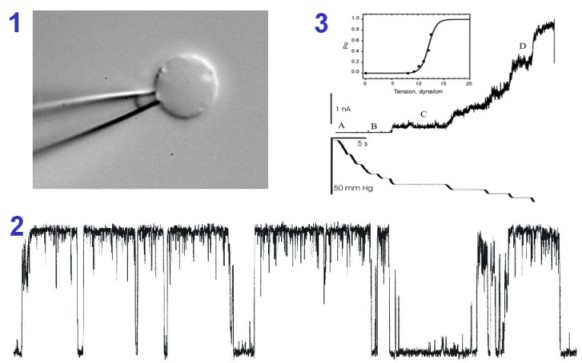
 | To characterize mechanosensitive channels
functionally we patch-clamp bacteria. For this purpose we generate
giant spheroplasts (1). MscL single-channel currents (2) are activated by
suction, they are large (70 pS at 20 mV) and display subconducting states.
MscL survives solubilization, purification and reconstitution into
liposomes. Panel 3 shows the activation curve recorded at different membrane
tensions from a multi-channel liposome patch (from Sukharev,
Sigurdson et al., 1999).
|
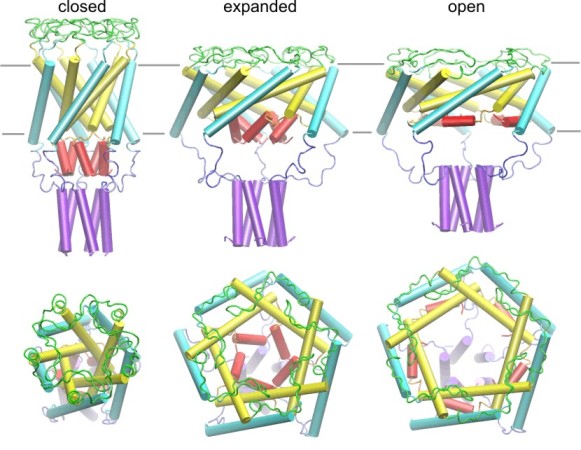
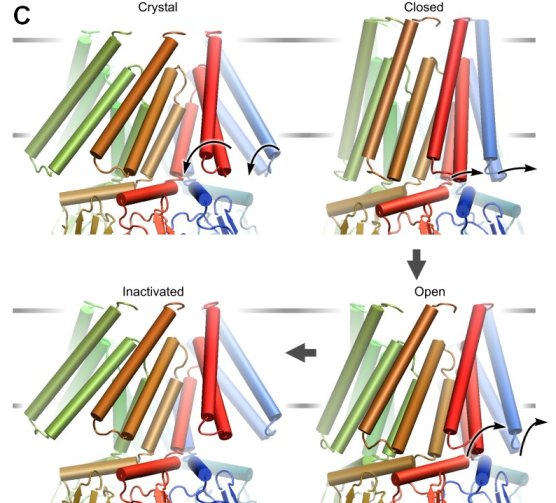
 | Models of the E. coli MscL in the closed,
expanded and open states created in collaboration with Dr. H.R. Guy. The
model of the closed conformation was generated by homology after the crystal
structure of MscL from M. tuberculosis (Chang,
Spencer, et al., 1998). The expansion of the barrel is achieved by
tilting and iris-like motion of the transmembrane helixes M1 (yellow) and M2
(cyan). The existence of a pre-expanded intermediate state was predicted
from the kinetic analysis (Sukharev,
Sigurdson et al., 1999). The helical structure of S1 domains (red) was
inferred from the amphipathic sequence of N-termini, which were unresolved
in the crystal structure. While the barrel expands and flattens, the
C-terminal helices (purple) remain associated in all conformations.
|
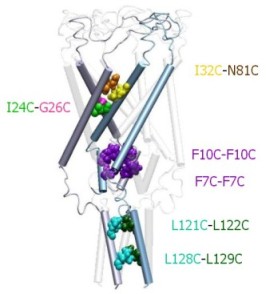
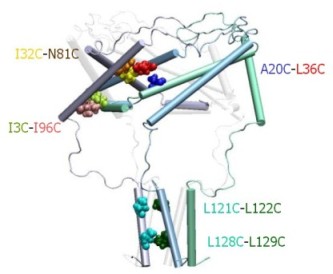
 |
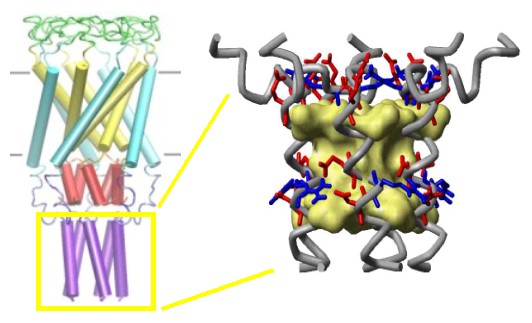
Structure of the C-terminal domains
of E.
coli MscL modeled after the crystal structure of the 5-fold
coiled-coil protein COMP. The helical interaction is
stabilized by apolar interactions of conserved aliphatic
residues inside the bundle (yellow surface), and multiple salt
bridges on the periphery.
|
 |
The molecular dynamic simulation shows that when the
ends of linkers are pulled radially to the predicted positions corresponding
to the open state, the C-terminal bundle remains stable. From Anishkin
et al., 2003. |
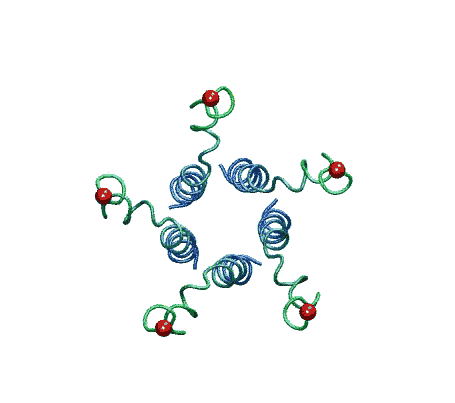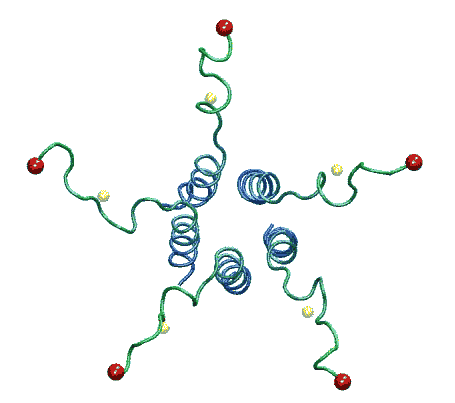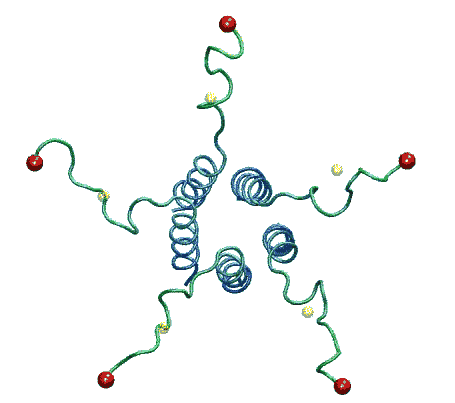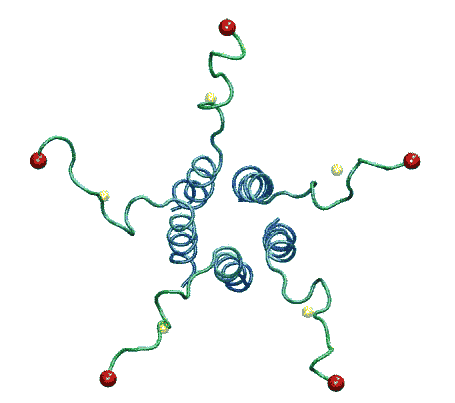
 | The model of the open state of MscL with the 'hanging basket' of
associated S3 helices. We propose this arrangement makes a pre-filter at the
cytoplasmic entrance, excluding high-molecular-weight substances from
entering the pore. |
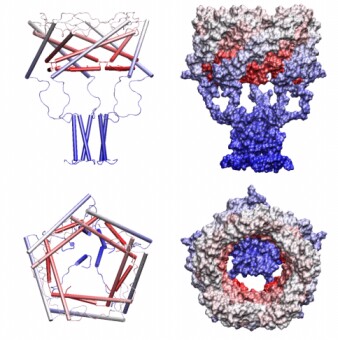
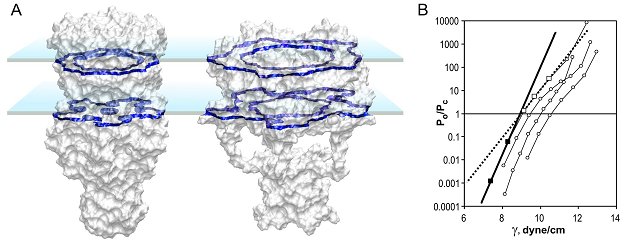
 | Determination of the MscL protein expansion. (A) Models of the closed
and open conformations shown with solvent-accessible surfaces predict the
mean in-plane expansion of 23.3 nm2. Blue planes represent the
boundaries of the hydrocarbon slab. (B) Activation curves measured on
multi-channel patches display inflections due to a slight non-homogeneity of
channel populations. The initial slope of left-most parts of the curves
predicts area and energy change DeltaA=20.4±4.8 nm2 and DeltaE =
51±13 kT as gross thermodynamic parameters for the gating transition (Chiang
et al., 2004).
More information (slideshow)... |
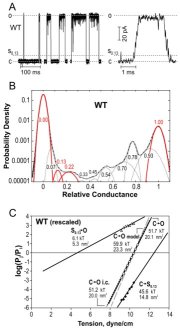
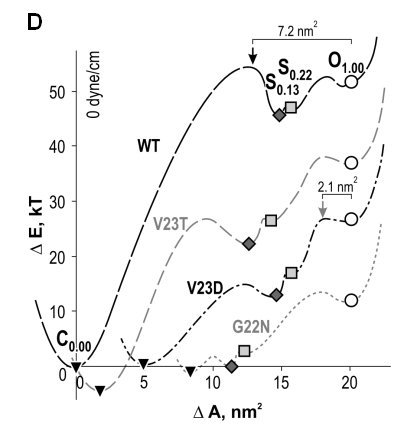
 | Gating patterns and analysis of WT MscL and
gain-of-function (GOF) mutants. (A) Raw trace with expanded fragment
illustrating the substate structure. (B) Amplitude histograms fitted with
eleven
Gaussian distributions defining probabilities for individual states; the
peaks for the closed (C), open (O) and two low-conducting states (S0.13
and S0.22) are shown in color. (C) Plots of probability ratios
for the pairs of C, S0.13 and O states as functions of
tension. (D) Spatial and energetic parameters of major substates,
extracted from such tension dependencies allow partial reconstruction of
gating energy profile for WT and GOF mutants (Anishkin
et al., 2005).
More information
(slideshow)...
|
|
(Click picture to zoom) |
 |
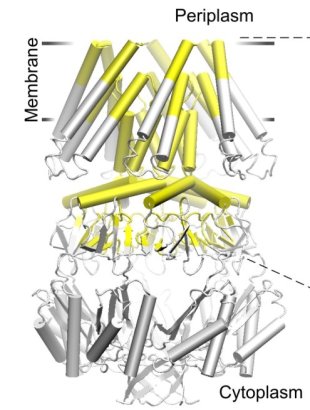
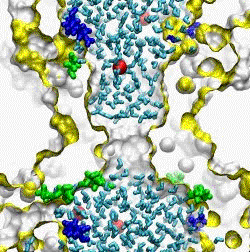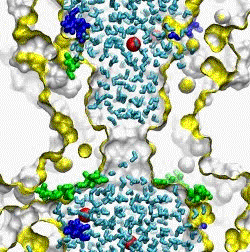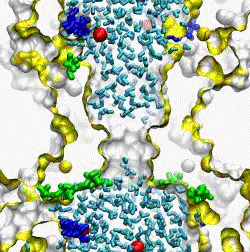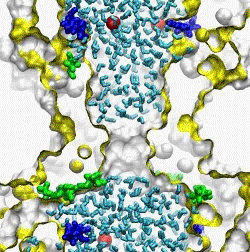
 | The crystal structure of the small conductance mechanosensitive channel
(MscS) has been an invaluable tool in the search for the gating mechanism,
however many functional aspects of the channel remain unsettled.
The pore constriction and outer chamber of MscS are lined with hydrophobic
sidechains L109, L105, A102, and A98 respectively. Hydration
energy estimations, as well as molecular dynamics simulations reveal strong
tendency to dewetting. We propose that the crystal structure represents a
non-conductive inactivated state of the channel (Anishkin
and Sukharev, 2004). Both inactivated and hypothetical closed states of
the channel are likely occluded by a "vapor
lock".
More information
(slideshow)... |
 |
 |
 | MscS displays peculiar channel kinetics characterized by
tension-dependent activation, and voltage-dependent inactivation occurring at
intermediate tensions (A). Thermodynamic and kinetic analysis of MscS
patch-clamp recording allowed us to reconstruct the main features of its
energetic landscape in two dimensions representing the "expansion area" and "transferred
charge" (B). Based on the extracted expansion and
gating charge values, as well as on the pore dewetting simulations we
proposed the gating scheme of MscS and currently developing structural models using
steered molecular dynamics simulations (C) (Akitake
et al., 2005).
More information (slideshow)... |
 |






