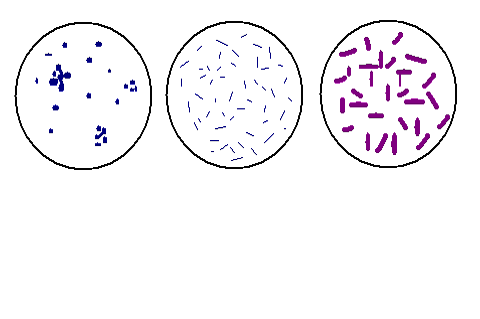L. casei is microaerophilic;
therefore, we left two of our jars unsealed while the third was sealed
with parafilm in order to create anaerobic
conditions. We used tap water to dilute our orange juice concentrate.
In jar one, the sealed jar; we used seventy milliliters of concentrate
with thirty milliliter of water. In Jar two we used 100ml of concentrate
in 700ml of water. In jar three we used 200ml of concentrate in 600ml of
water. We left all the jars at room temperature for one week. After one
week we extracted samples from all three jars. We made sure to take our
samples from all different locations in the jars ranging from the top slime
to the bottom gunk. We streaked three plates from each jar; extracting
different specimens from different locations for each plate we streaked.
 |
Constituents of Lactobacillus Selection Oxgall Agar
Sodium Acetate * 3H2O 25.0g
Dextrose
20g
Agar
15g
Pancreatic Digest of Casein 10g
KH2PO4
6g
Yeast Extract 5g
Ammonium Citrate 2g
Oxgall
1.5g
Polysorbate 80 1g
MgSO4
.575g
FeSO4
.034g
MnSO4
.12g
Acetic Acid, glacial 1.32mL
|
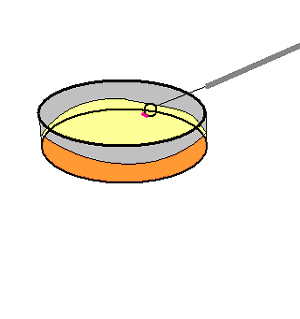
We streaked the extracted specimens onto our Oxgall
enrichment plates. After streaking the specimens onto the Oxgall plate
we tightly sealed the plates with parafilm
and left them at room temperature.
After five days we observed growth on all nine plates.
We looked for smooth glistening, opaque color colonies with no odor. We
scanned our plates and used one plate from each jar as possible isolations
of Lactobacilluscasei.
We then proceeded to conduct gram stains for each
of the specimens we derived from all three plates. We were looking for
rods with square ends, possibly in chains, or vibrio
shaped in our samples. Through our gram stains we were able to pinpoint
exactly which sample had our organismís characteristics.
Jar 2 seemed to have the organism we were looking
for. After we conducted our Gram Stains we preceded to streak plate our
desired organism from jar 2 once more in order to get pure culture on our
LSO agar. After determining that our plate was a pure culture by both observing
the characteristics of growth on the LSO plate and observing the gram stain
we conducted the Catalase test on our specimen. Lactobacillus casei
is Catalase negative and therefore we were expecting to see no bubbling
when Hydrogen Peroxide was added to the sample. Our sample had no bubbling
so therefore we determined that is was Catalase negative and it was possibly
Lactobacillus
casei.
Confirmation Process:
In addition to the Catalase
test for confirmation we decided to use the mannitol
test, motility stab, oxidation fermentation tube test as well as ADT-CaCO2
agar plate. We conducted all of these tests on the same day using L. casei
from the same inoculated plate. All of our confirmation tests were run
in room temperature. All of the tests were made by the microbiology department.
Return To
Index