Equilibrium
Equilibrium by
definition is the state of a chemical reaction in which its forward and reverse
reactions occur at equal rates so that the concentration of the reactants and
products does not change with time; however, in the living organisms,
equilibrium is hardly achieved since the condition is always changing.
An example of the
equilibrium would be: A D B when µA = µB
or Keq = ![]() …i.e. the concentrations of A
and B are at their equilibrium values.
The energy differences between A and B are compensated for by the
differences in concentration.
…i.e. the concentrations of A
and B are at their equilibrium values.
The energy differences between A and B are compensated for by the
differences in concentration.
Another example of
equilibrium is equilibration between compartments:
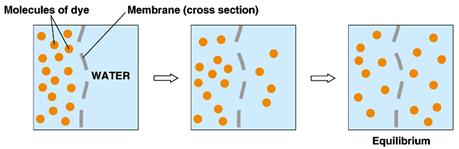
Here µA (left) = µA (right)
…that does not mean that [A] (left) =[A] (right) …as in the
picture. The chemical potential energy
level is the same on both sides.
Steady State:
An Example of the Steady
State: the movement from A à X à B continuously; however, no changes occurs to [X] because X is formed
from A as fast as it is converted to B.
thus![]()
…no
change in a concentration does not mean equilibrium. Cells typically
have constant values of solute concentrations, such as pH, and transmembrane
voltage but these do not represent equilibria.
Another example of steady
state in cells is that the total current (I) through the membrane is equal to
0. That must be true if the
transmembrane voltage is constant. For example the potential inside a cell
is about -10mV and if the potential stays constant, then the current through
the membrane is equal to 0…more about this later.
The Chemical Potential
The chemical potential of a
substance is a great starting point.
From this we can get many familiar relationships.

![]() µA = µºA + RT ln[A] + ZAF φ + VA P
µA = µºA + RT ln[A] + ZAF φ + VA P
F If A D B then Keq = ![]() =
= ![]()
You can look at this as an
application of the Boltzmann distribution.
Even though B and A are different molecules they
can be looked at different forms of matter at different energy levels. Thus, at equilibrium, the ratio of [B] over
[A] should be given by the energy difference and the Boltzmann distribution. Alternatively, we can use the chemical
potential. At equilibrium:
µA = µB è µºA - µºB = - RT ln![]() where µºA - µºB = ∆Gº
where µºA - µºB = ∆Gº
The symbols with the superscript º
contain the information of the inherent energy of A and B that has to do with
the nature of the substance and the environment in which the substance
lies. When the reaction is not at
equilibrium, there is a tendency for the reaction to move toward equilibrium
and thus there is an energy difference between reactants and products. This energy difference is the ∆G. It depends on how far the reaction is from
equilibrium. [A] and
[B] are the actual concentrations.
∆G = - RT ln![]() + RTln
+ RTln ![]() = ∆Gº + RTln
= ∆Gº + RTln ![]()
F If A D B + C then we know
that ![]() but how do we write
the Boltzman distribution?
but how do we write
the Boltzman distribution? 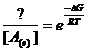
If we use the chemical potential equations
it becomes clear:
µA = µB + µC ….at equilibrium the
energies must equal…energies add
è µºA
- µºB - µºC = ![]()
converting to the exponential form….
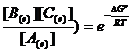
So we see that in the Boltzmann distribution
the concentrations…or the probabilities…should be multiplied. That makes sense because the probabilities
are always combined by multiplication.
Example of energy changes in a reaction and how these depend on distance
from equilibrium:
In
a typical cell: ATP + H2O D
ADP + Pi and thus:
![]()
*H2O is
not used in the equation because H2O is
pure.
ATP + H2O D ADP + Pi ∆Gº = -31kJ/mol
The negative ∆Gº tells us
that the reaction favors the products....can you tell why?
Hint: from ∆Gº one can
determine the equilibrium concentrations.
Important: The
energy available from ATP hydrolysis is NOT fixed but depends on distance from
equilibrium.
Example of the use of energy by
cells to promote a reaction that is not favorable…i.e. the energy of the
products is greater than that of the reactants.
The first reaction in glycolysis…the use of sugar by cells…needs to
phosphorylate glucose:
![]()
![]() Glucose +
Pi
Glucose-6-Phosphate ∆Gº =
+ 14kJ/mol
Glucose +
Pi
Glucose-6-Phosphate ∆Gº =
+ 14kJ/mol
Note the positive ∆Gº….lets call this by
its name: the standard change in free energy…but please remember what this
really means.
The ratio of products to reactants at
equilibrium is 3x10-3 …not a great start…good if you are on a diet
Cell’s solution: combine this unfavorable
reaction with the hydrolysis of ATP
The addition of the two reactions gives rise to
a spontaneous reaction:
ð
![]()
![]() Glucose + ATP
ADP + Glucose-6-Phosphate ∆Gº
= - 17KJ/mol
Glucose + ATP
ADP + Glucose-6-Phosphate ∆Gº
= - 17KJ/mol
The enzyme, hexokinase, couples these reactions
together. This is only way in which
energy is used to shift a process in a non spontaneous direction.
NOTE: this is not the full story because we are
dealing with ∆Gº not ∆G. The
real free energy change depends on the concentrations of reactants and
products. The cells
keeps the [ATP] about 100 times higher than the [ADP]. Therefore the energy difference for the
reaction out of equilibrium is much greater.
Question: How
is it that the energy available from ATP hydrolysis depends on how far the
system is from equilibrium while the energy released from burning fuel
(gasoline, oil, wood) is constant?
These systems
are far from equilibrium! The reverse
reaction is negligible.
Cells work at
energies close to thermal energy and so many reactions are not far from
equilibrium.
Equilibrium between solutions
separated by a membrane:
Consider a bacterium swimming in
almost pure water. If we add some
glycerol to the medium so as to make it 1 mM, what might the concentration be
within the cytosol of that cell at equilibrium?
What we need to know:
- Water easily crosses
membranes and reaches equilibrium.
- Glycerol also permeates
through membranes easily and reaches equilibrium.
- The concentration of solutes
in a cell dilute the water inside and thus when water reaches equilibrium
a pressure develops inside that is higher than outside.
![]() V’ is V bar…the
volume of a mole of water
V’ is V bar…the
volume of a mole of water
at equilibrium: ![]()
Thus a pressure difference is generated to
balance the concentration difference of water.
The pressure difference affects all the molecules, including the
glycerol.
at equilibrium: ![]() ….V’ is the volume of a mole of glycerol.
….V’ is the volume of a mole of glycerol.
Glycerol