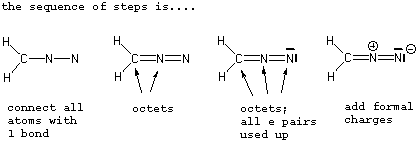1 . Must start with the correct connectivity...this is essential
2 . Add up the total number of valence e's from all the atoms in the molecule and divide by 2 to obtain the number of electron pairs. If the species has a + charge, subtract 1 e from the total and if it has a - charge, add one to the total. See C below.
3 . Fill in the structure with the appropriate number of lines (e pairs from # 2) so that all atoms (such as C, N, O ,X) except H have satisfied octets. Start by connecting all atoms with a single line (the sigma bonds) and then use up the remaining lines to give all atoms octets.
4 . Finish up by designating formal charges where appropriate.
Things to watch out for....
A . If the species contains a group I or II element, such as sodium or magnesium, represent these species as cations, such as Na+ or Mg+2 .
B . In the case of B, there may not be enough e's to given B an octet. This would be the case in BH 3.
C . If the species has an odd number of e's, dividing by 2 will give a quotient that is not an integer, such as 8.5. This means that 8 lines are available plus 1 unshared e. In this case, all atoms will not be able to have satisfied octets. Most often happens with atoms that have an odd number of N's; example is NO 2.
Examples....
NO 2 sum of valence e's = 5 + 2 x 6 = 17; # e pairs = 17/2 = 8.5

MgCO 3 sum of valence e's = 2 + 4 + 3 x 6 = 24; # e pairs = 24/2 = 12

CH 2NN sum of valence e's = 2 x 1 + 4 + 2 x 5 = 16; 16/2 = 8 lines or e pairs