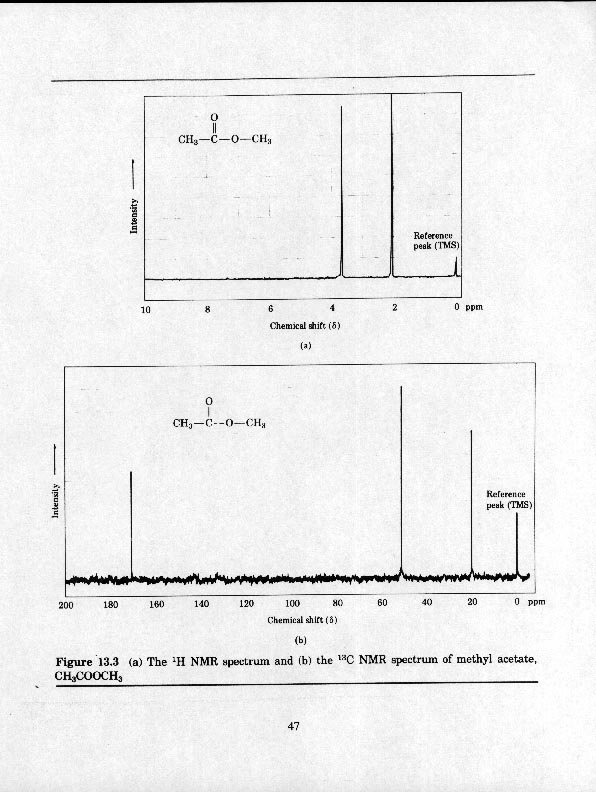
13C NMR NUCLEAR MAGNETIC RESONANCE
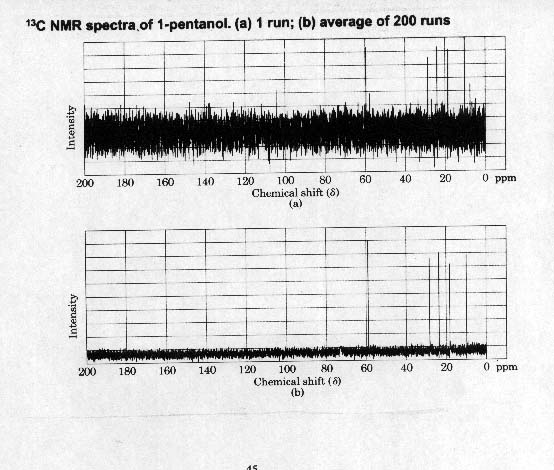
Unlike proton NMR in which the isotope observed is the most naturally abundant, in carbon NMR we observe C-13 which accounts for only 1.1% of carbon. This problem is overcome by 1) signal averaging and 2) Fourier-transform NMR.
1) In signal averaging several hundred or thousand runs are made and added together and then averaged. The random noise background averages to zero and the signals become prominent.

2) Fourier-transform speeds up the process. Sample is placed in magnetic field and irradiated with a pulse of all useful frequencies giving a complex signal that is mathematically manipulated so that the spectrum is shown in the normal manner. This mathematic manipulation is called Fourier-transform.
1. Number of signals
As with proton NMR, each distinct carbon has a signal and the number of signals shows how many differecnt kinds of carbon are present in the molecule.
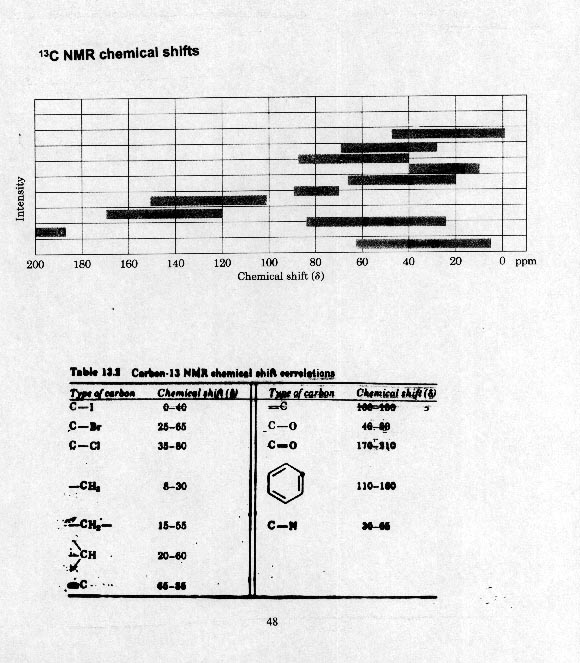
2. Chemical shifts - general trends
As with protons, electronegative elements cause downfield shifts. So carbons attached to oxygen, halogens, etc. appear downfield from normal alkane carbons.
Hybridization plays a role in shift also. sp
Carbonyls are quite far downfield - acids, amides, esters 175-200
d and aldehydes and ketones >200 d.3. Integration
Peak size in carbon NMR is not always a function of how many of each kind of carbon are present. Other factors influence this. In some newer instruments can get an integration. Will not worry about this.
4. Spin-spin coupling
Since
-CH
3 - quartet-CH
2 - triplet-CH - doublet
-C - singlet
Generally run "broad-band decoupled" 13C spectra and see only singlets. There are methods to determine the number of protons on each of the carbons.