Plecotus auritus
The Brown Long-Eared Bat
Taxonomy
Plecotus auritus, also known as the brown long-eared bat, belongs to
the family Vespertilionidae of the order Chiroptera. Specifically, the species
is classified among the microchiroptera bats.
Geographical Distribution
The species is widespread in
Europe, with the exception of southern Spain, southern Italy, and Greece. It is
the second most common species of bat in Britain, although it is absent from
north and northwest Scotland. The
animal can also be found in India, Nepal, Mongolia, Siberia, China, Sakhalin,
Japan, South Korea, and the Himalayas.
Physical Characteristics
The average head-body length is approximately 37 to 48 millimeters, and
the wingspan ranges from 230-285 millimeters. The body weight of the animal
normally ranges from 5 to 12 grams. The females of the species are slightly
larger and heavier than the males. The adult bat has brown fur with a pale
underside and a pink-brown face, while juveniles are more gray in color
overall. P. auritus have relatively large eyes, and
slit-shaped nostrils that open laterally. The
most distinguishing features are the animalıs ears, which are nearly the length
of its whole body. When extended, the ear can be up to 25 millimeters
long. At rest, their ears are
tucked back and folded, resembling ram horns. When the animal hibernates, the
ears are tucked back so far that only the tragus is visible.
Habitat
The general habitat of P.
auritus is in sheltered valleys and
mountainous woodland. In the summer, the species roosts in barns, hollow trees, and old buildings. In
the winter, it can be found hibernating in caves, mineshafts, hollow trees, and
underground sites.
Roosting Behavior
Roosts provide the bats
shelter from the weather, protection from predators, and a warm environment for
rearing their young. Colonies typically consist of 10-20 adults, but can
contain as many as 50 individuals. Entwistle (1994) found that in May, the bat
colonies of the species were nearly all female, but the number of males
increased from June to September with a decease in the number of females and
juveniles. By October, males dominated the colonies. However, females tend to
remain in one roost all their lives, while males are more likely to depart to
another roost.
Diet and Foraging Behavior
The species is insectivorous,
and feeds primarily on moths. They also eat caterpillars, spiders, earwigs,
dung beetles, and flies. They forage at night, usually relatively close to
their roost. Foraging usually ends after about 4 or 5 hours. The bat catches
insects in flight or by foliage gleaning - picking insects out of trees or off
the ground. Gleaning is advantageous over aerial capture because moths cannot
as easily detect the bats presence so gleaning bats eats more moths than do
aerial hunters. Additionally, gleaners are less dependent on air temperature
and also they can catch non-flying prey like spiders and caterpillars. Aerial
hunters catch insects directly in their mouth, but also can use the tail and
wing membranes to scoop up moths or corral the insect toward the mouth. Also,
Plecotus auritus sometimes perform
steep somersaults techniques to catch prey. Plecotus bats not only fly in straight horizontal paths, but
also are known to hover, especially when gleaning for food from vegetation or
tree trunks. Figure 1 represents each type of flight.
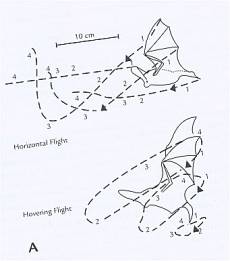
Fig 1. Flight of the long-eared bat, Plecotus auritus. The position of the wings at each
of four 10-ms intervals is indicated by the numbers 1-4 on the dashed line.
This diagram represents the path taken by the wings during horizontal flight
(top, flight speed = 2.35 m/s) and hovering flight (bottom, flight speed = 0). (Norberg, 1976)
Hibernation
Prior to hibernation, in late
summer, the bats accumulate fat and this is used as an energy store for the
winter. Hibernation begins in November and ends in late March. When the bat is hibernating, the wings are folded
around its body, with its ears tucked under. Plecotus auritus typically hibernates in caves, tunnels, mines, buildings, and tree
holes. The animals prefer to hibernate at very cold temperatures, just above
freezing. They hibernate solitarily or in small groups. Premature disturbance
of hibernating bats can cause decreases in their population because it arousal
causes them to use up energy resources and in consequence they may run out of
energy and die before the end of winter. As a result, biologists and tourists
are restricted in how they observe the bats.
Mating Behavior
The male brown long-eared bat
has facial glands which produce a
brown, odorous secretion used to mark potential mating roosts. The male visits
as many roosts as possible to maximize its chances of mating, and marking
roosts helps to advertise their presence in order to attract females. P.
auritus is considered to show random,
promiscuous mating, where swarming occurs and females mate with many males.
Reproduction
The reproductive cycle of the brown long-eared bat is long - spermatozoa produced in one summer do not result in live young until the following summer. Males produce the greatest amount of sperm in late August/September and the mating period typically begins in October. Mating continues sporadically throughout the winter, but the maleıs sperm production ceases in November. Females delay fertilization until late April or May, after hibernation ends. This is when the female begins ovulation and sperm stored in the uterus fertilizes the ovum. The females then move into nursery roots (maternity colonies) and parturition takes place, usually in early July. The female gives birth to only one offspring per year (the length of one breeding season); twins are very rare. Because the reproductive cycle is long, complex, and only yields one infant, a female whose baby dies loses it chance to reproduce for a whole year. Fortunately, the species has evolved adaptations to minimize such failures, and studies have found that 70% (Entwistle, 1994) to 97% (Benzal, 1991) do produce young each year.
Long-eared bats are born
hairless, pink in color, and with their eyes closed. In the first week of its
life, the infant bat clings to its mother nipple. The baby is born with
incisors that have hooked tips to enable the animal to grip the nipple, even when
the mother is in flight. When the mother forages, the newborn bat is left
clinging to beams in the roost. By 4 days old, the baby has a covering of hair
and at 6 days old, the ears become erect and the eyes open. By 10-12 days,
babies begin to groom themselves and flap their wings. After 20 days, they can
fly around the roost, and by day 35, they first leave the roost on their own
wings. Weaning of the infant is complete about six weeks after birth.
The male Plecotus auritus reaches sexual maturity in the second summer of their
life. The female is sexually mature anywhere from 24-36 months of age. The
maximum age for the species is 30 years, but the average life span is seven
years for males, sixteen for females.
Echolocation
Because the echolocation
calls of Plecotus auritus are very
quiet, these bats are known as "whispering bats". I. Ahlen (1981)
described the calls as faint and short FM sweeps, about 2 ms long, and with
prominent second harmonics. Using quiet calls is beneficial to the animal
because the prey is less likely to detect its presence. The frequency of the
brown long-eared batıs call is usually 83-26 kHz, but sometimes louder at 42-12
kHz. Figure 2 represents a loud-long sweep produced by Plecotus auritus.
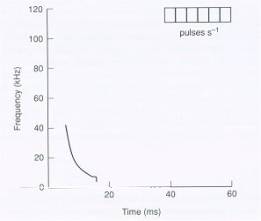
Fig 2. Diagrammatic representation of the loud-long
sweep (Ahlen, 1981) of P. auritus
In general, CF signals, which increase the range of target detection, are used by the bats for hunting in open areas, while FM signals allow them to more accurately locate obstacles and targets in cluttered environments. Hence, gleaning bats use short, low-intensity FM signals to distinguish between insects and foliage while foraging.
Predation and Parasites
The habit of the brown
long-eared bat to fly close to the ground makes it vulnerable to attack by
predators, especially the domestic cat, Felis catus. Other predators include the barn owl (Tyto alba), the tawny owl (Strix aluco), and the kestrel (Falco tinnunculus).
Plecotus harbor relatively few ectoparasites, especially the
adults. This is believed to be connected with small colony size in these bats,
since fleas and mites cannot be transferred as easily. Also, the batıs tendency
to move from roost to roost further reduced the opportunity for parasite
populations to increase. As for internal parasites, Gardner et al. (1987) examined blood smears of P. auritus and found only one bacterium, Grahamella, which is transmitted by fleas and not harmful to the
host.
Relationship With Humans
P. auritus is considered to be very docile and one of the
easiest European species to handle and to tame. Their lives are greatly
entwined with those of human beings, as they utilize human homes and mine
shafts for shelter and protection. However, humans are more of a threat than a
positive influence to these bats. The most serious problem this species faces
is the loss of deciduous woodland due to large-scale farming. Other threats
include barbed wire, which bats can be impaled upon during foraging, deliberate
destruction of roots by pest exterminators, and disturbance from hibernation.
Plecotus auritus is not considered to be an endangered species, but
that does not mean it is without protection under the law. In Britain, bats are
protected under the Wildlife and Countryside Act of 1981, which states it is
illegal for anyone without a license to intentionally injure, kill, or even
handle a bat, to possess or try to sell a bat, or to disturb a roosting bat.
Europeans even set up bat boxes, or artificial roosts similar to bird nesting
boxes, for the bats to use. These bat boxes are popular with both the public
and bat enthusiasts because they are a practical method of protecting bats and
keeping them from intruding human homes.
Bibliography
Ahlen, I. (1981). Identification
of Scandinativan bats by their sounds.
Report No. 6, Dept. Wildlife Ecol. Swedish Univ. Agric.Sci.
Altringham, John D. Bats:
Biology and Behavior. New York: Oxford University Press Inc, 1996
Benzal, J. (1991). Population
dynamics of the brown long-eared bat (Plecotus
auritus) occupying bird boxes in a pine forest planation in central Spain. Neth. J. Zool., 41 (4): 241-249
Entwistle, A.C. (1994). Roost
ecology of the brown long-eared bat (Plecotus
auritus) in northeast Scotland.
Unpublished PhD thesis, University of Aberdeen, UK
Fenton, M. Brock, Just
Bats. Toronto: University of
Toronto Press, 1983
Gardner, R.A., Molyneux, D.H.
and Stebbings, R.E. (1987). Studies on the prevalence of haematozoa of
British bats. Mamm. Rev., 17 (2/3):
75-80
Neuweiler, Gerhard. The
Biology of Bats. New York: Oxford University Press Inc, 2000
Norberg, U.M. (1976). Aerodynamics,
kinematics, and energetics of horizontal flapping flight in the long-eared bat, Plecotus auritus. J. Exp Biol. 65:179-212
Norberg, U.M. (1976). Aerodynamics
of hovering flight in the long-eared bat, Plecotus auritus. J. Exp Biol. 65: 459-470
Nowak, Ronald M. Walkerıs
Bats of the World. Baltimore: Johns Hopkins University Press, 1994
Swift, Susan M. Long-Eared
Bats. London: T&AD Poyser Ltd, 1998