Research
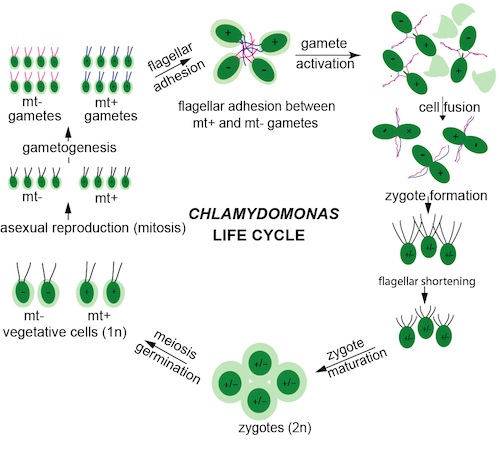
Fundamental elements of ciliary signaling arose early in evolution and are critical for key developmental events in unicellular organisms, indicating that signaling pathways have not migrated to the cilium during evolution; rather, they never left the organelle. Moreover, the key events in fertilization, gamete recognition/adhesion and fusion of gametes to form a zygote define sexual reproduction in unicellular and multicellular organisms. Our laboratory uses Chlamydomonas fertilization as a model system to dissect fundamental mechanisms of cilium-generated signaling, ciliary/flagellar length control, and membrane fusion reactions during cell-cell fusion/zygote formation. Chlamydomonas gametes of opposite mating types (plus and minus) are endowed with adhesion and signal transduction molecules on their flagella (SAG1 on plus and SAD1 on minus) that enable tem to bind to each other to initiate a signal transduction cascade, including several protein kinases and an adenylyl cyclase. The consequent increase in intracellular cAMP triggers biochemical and morphological events (collectively termed gamete activation) that prepare the gametes for cell-cell fusion and that also mobilize more of the adhesion molecules to the flagellar to enhance and maintain flagellar adhesiveness and signaling capacity. Gametes activate specialized fusogenic regions between their two flagella, the mating structures, and in the second phase of fertilization, the two gametes also adhere to each other at their mating structures. The membrane fusion reaction quickly follows and the two gametes coalesce into a single diploid zygote (the equivalent of a fertilized egg in multicellular organisms). Gamete fusion almost immediately downregulates signaling, and soon after fusion the zygote shortens its flagella and prepares to undergo meiosis to begin the life cycle again.