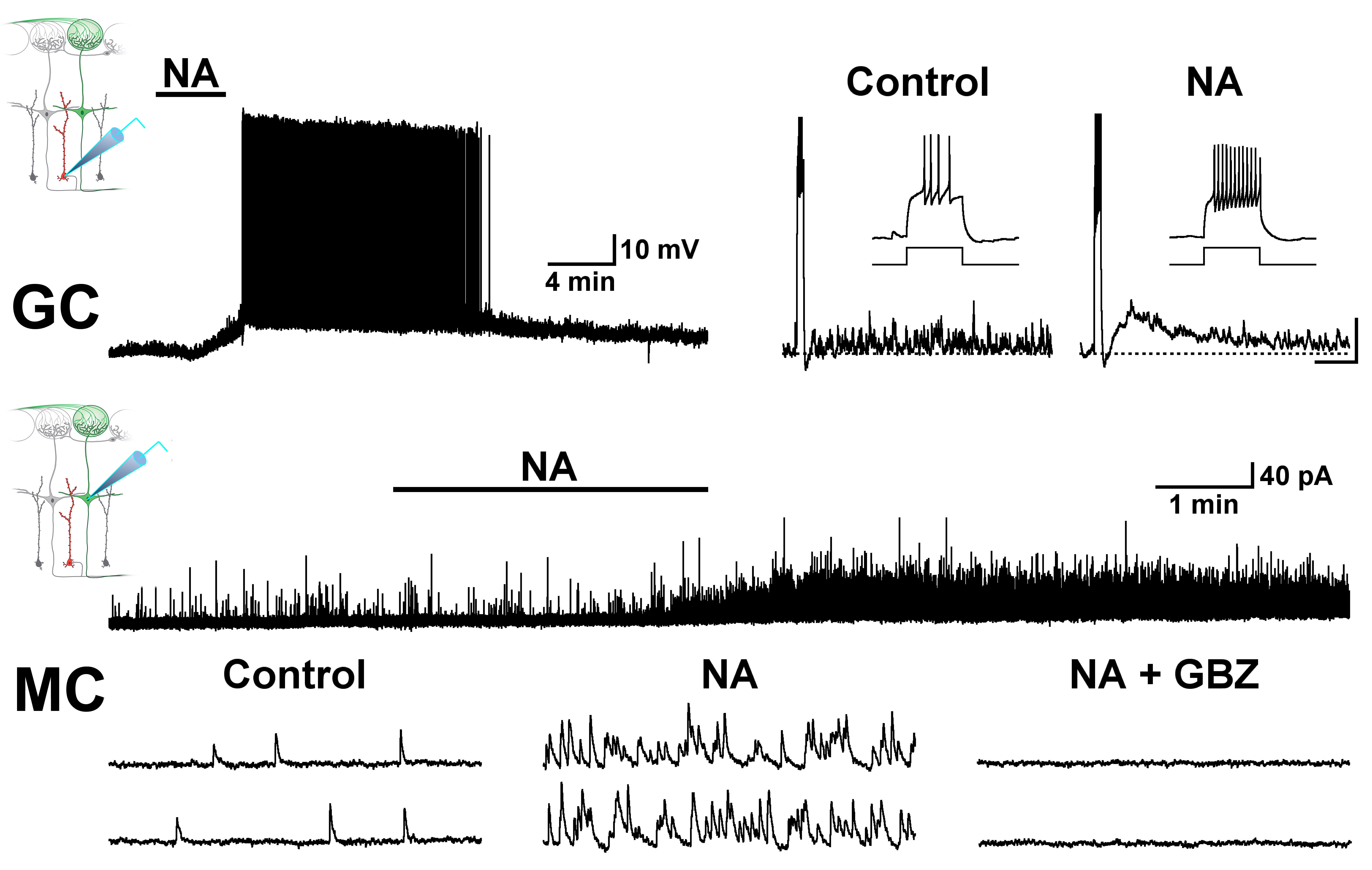
Neuromodulatory transmitters influence most neuronal processes including sensory gating, sleep-wake cycle and learning, while their dysregulation underlies a range of neuropsychiatric disorders including depression and anxiety. My long-term research goal is to understand how neuromodulatory transmitters such as noradrenaline and acetylcholine regulate neuronal activity in the brain.
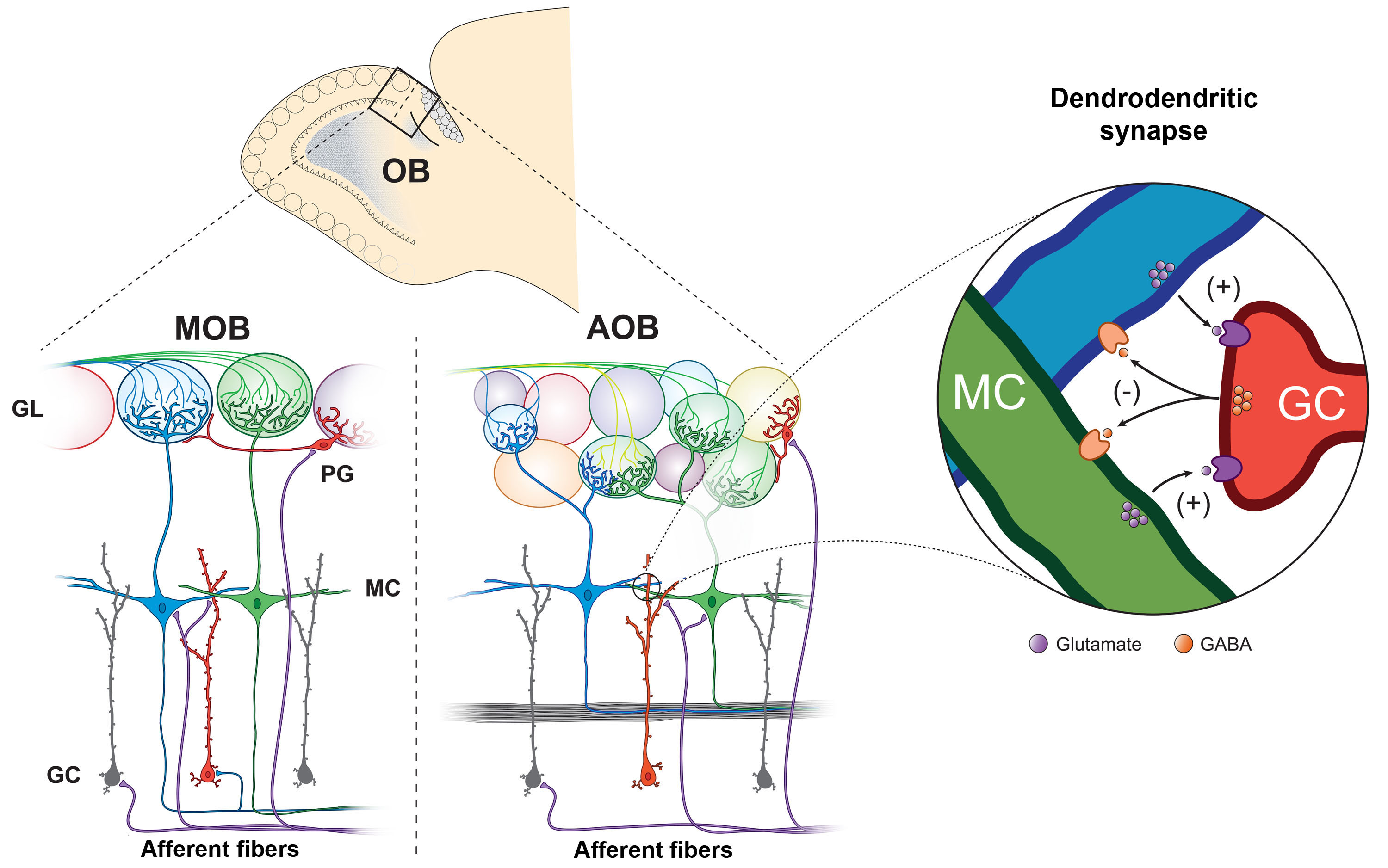
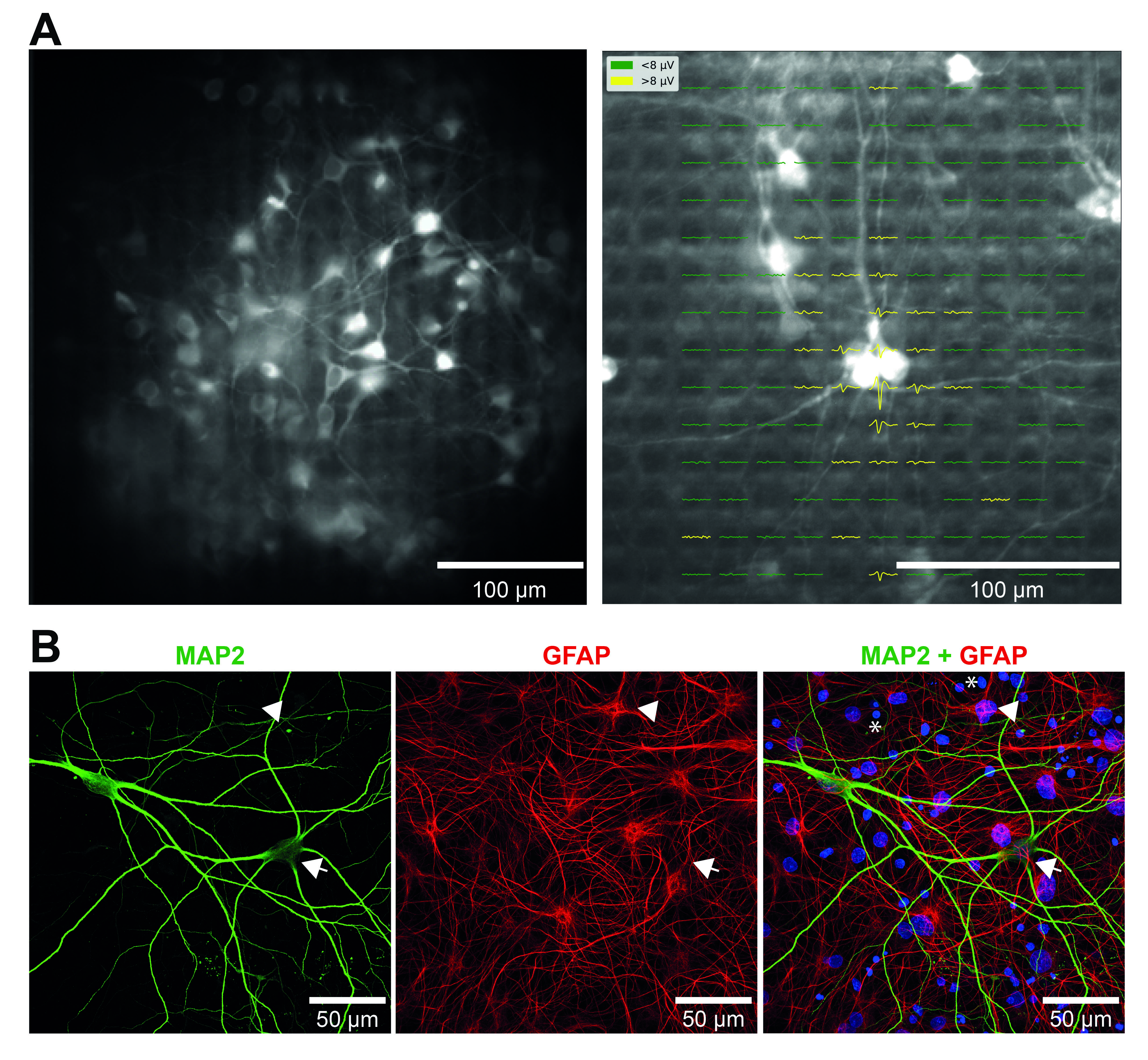
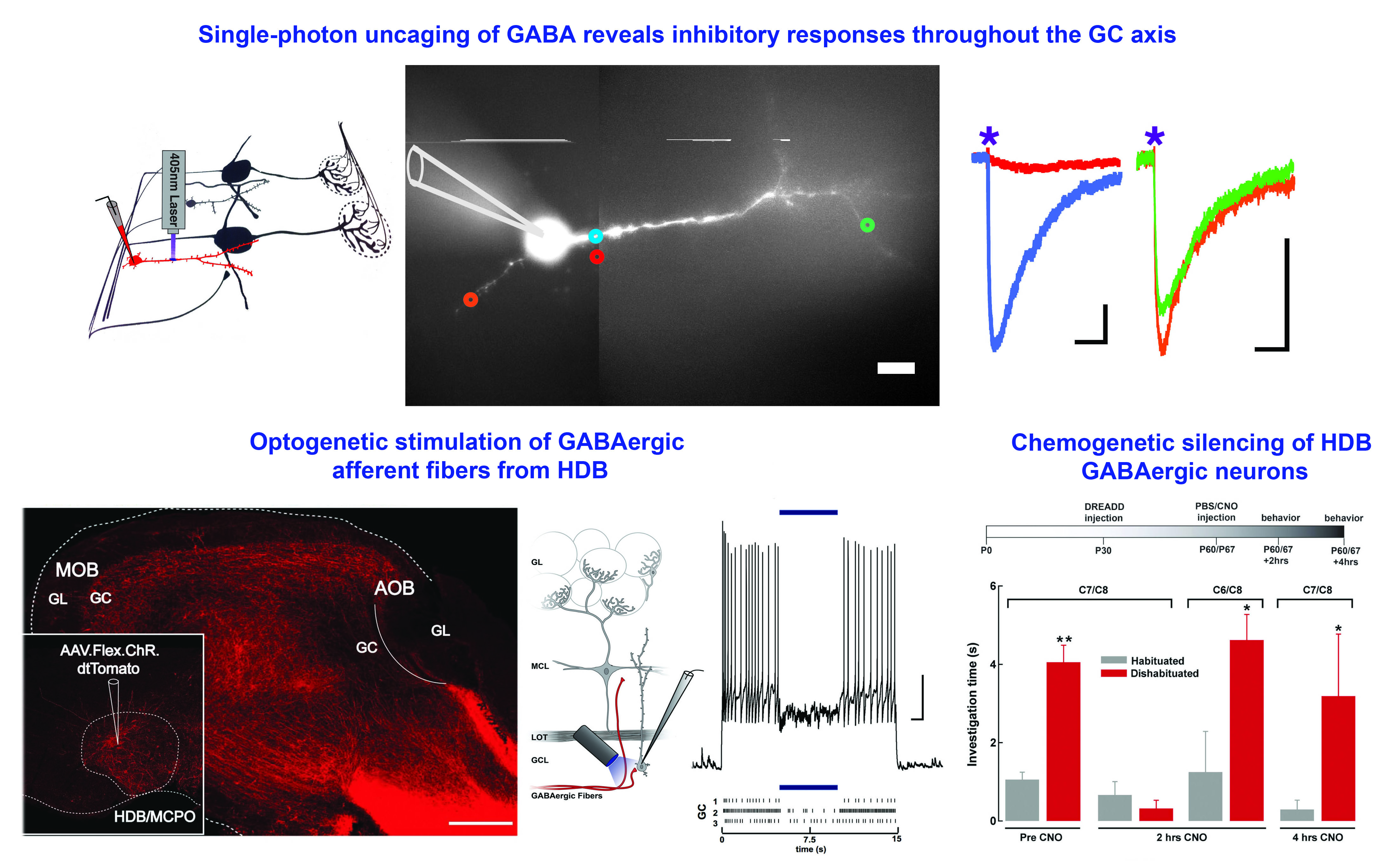
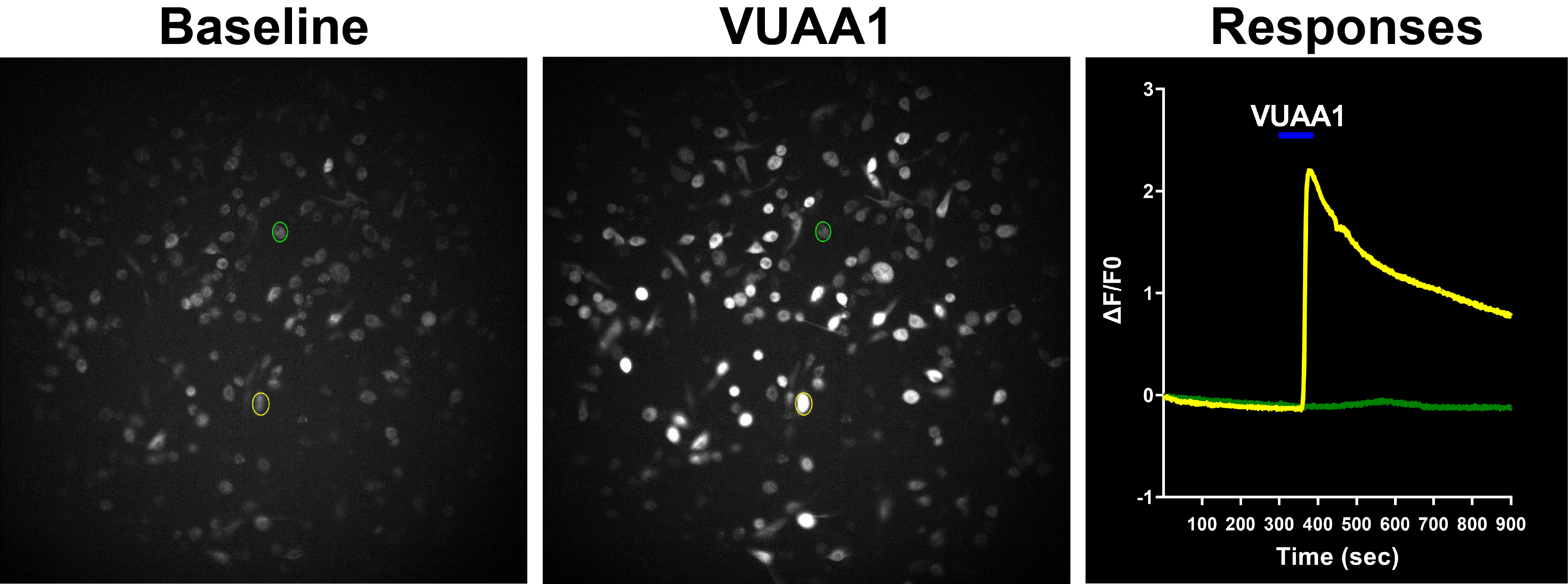
To learn how neuromodulators influence sensory processing and behavior our lab uses olfaction as a model system Currently, our research focus is on the olfactory bulb (OB), the gateway of odor information into the brain, and the first area where odor processing occurs. Specifically, we use targeted expression of optogenetic and chemogenetic tools to precisely control the activity of neuromodulatory neurons that regulate the OB circuit, and learn how this regulation influences odor-guided behaviors.
Exceptionally, the OB is among the few neural circuits known to incorporate adult-born neurons in the brain. Thus, another research area in our lab addresses how neuromodulation and odor-guided behaviors drive the integration of inhibitory neurons into preexisting circuits of the adult brain. Our research advances the understanding of sensory and cognitive functions while providing fundamental translational information for the development of therapeutic strategies that combat brain disorders.
In addition to research, a rewarding aspect of academic life is the teaching and training of young scientists. In the past years, I had the privilege of mentoring a number of bright and promising minds, including 35 undergraduate, 6 graduate and 5 high school students. Please visit the Lab News page to learn about their achievements and current positions.