|
|
||||||
| Home | CBMG | Contact | Faculty | Graduate | Undergraduate | Research |
| ||||||||||||||
|
|
|
Macrophage heterogeneity Macrophages reside in virtually all tissue under a variety of names (kupffer cells, osteoclasts, microglia, etc). These cells have the remarkable ability to rapidly change their phenotype in response to different environmental stimuli. This plasticity allows them to participate in a variety of diverse host responses, including host defense, wound healing, and immunoregulation. Our research seeks to understand the molecular mechanisms that control these phenotypic alterations. We described a population of macrophages with potent immunoregulatory activity. These regulatory macrophages shut off several inflammatory cytokines and produce large amounts of the anti-inflammatory cytokine, IL-10. The addition of a small number of regulatory macrophages can prevent lethal endotoxemia in mice and can potently mitigate autoimmune pathology. We propose that regulatory macrophages arise to prevent the pathology that accompanies excessive immune responses. We are currently developing polypeptides that induce the generation of regulatory macrophages and testing them in animal models of autoimmunity.
Figure 1. Resident tissue macrophage hours after being washed from the peritoneum of a BALB/C mouse.
Leishmania and host defense The protozoan parasite, Leishmania spp. is transmitted to the human host from infected phlebotamine sandflys. Promastigote forms of the parasite (Figure 2) are delivered to humans when the fly takes a bloodmeal. Parasites rapidly take up residence in tissue macrophages. Within macrophage phagolysosomes amastigote forms of the parasite (Figure 3) replicate in macrophages and spread the infection to neighboring macrophages.
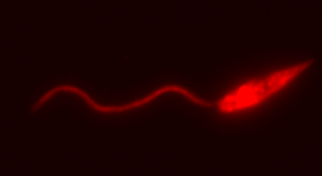
Figure 2. Leishmania major expressing red fluorescent protein, kindly provided by our collaborator Alain Debrabant. 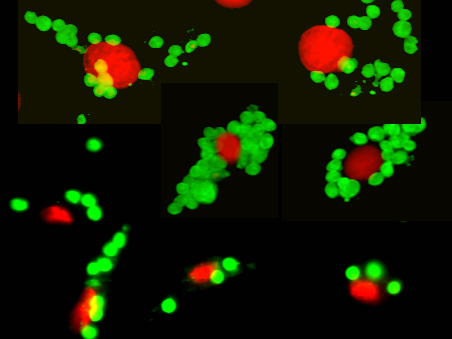
Figure 3. Leishmania amastigotes (green) growing inside macrophages. Macrophage nuclei are stained in red. The macrophage cytosol and plasma membrane are not visible in this photomicrograph. One of the hallmarks of macrophages is their ability to become “classically activated”. These classically activated macrophages are endowed with an increased capacity to kill intracellular microbes. Classically activated macrophages produce oxygen and nitrogen radicals to limit the intracellular growth of Leishmania parasites. We have undertaken studies to understand how macrophages become activated to kill these parasites and how the parasite manipulates the immune response to avoid this killing. Monocyte heterogeneity The term monocyte refers to a leukocyte that is produced in the bone marrow and released into the blood. Monocytes typically exit the blood for tissue where they differentiate into macrophages or dendritic cells. We now know that monocytes are not a single homogenous population of cells. Rather, monocyte subpopulations can differ with regard to size, granularity, and the expression of surface markers (Figure 4). These different monocyte subsets can have dramatically different functionality. The so-called “classical” monocytes are larger, more granular, and they express a chemokine receptor, CCR2 which allows them to rapidly exit the blood in response to inflammatory stimuli. The non-classical monocytes express high levels of the fractalkine receptor (CX3CR1) and they have been reported to have “patrolling” activity. We have examined these two major monocyte subsets (there is a third “intermediate” subset) for their ability to kill microbes. The classical monocytes produce high levels of superoxide and rapidly kill Leishmania major promastigotes in culture. The non-classical monocytes, in contrast, are much more susceptible to infection by L. major. Studies are underway to examine the transcriptomes of these monocyte subsets to identify their roles in host defense and homeostasis.
From: Strauss-Ayali, D. Conrad, S. and Mosser, D.M. 2007. J. Leukocyte Biol. 82:244-52. Figure 4. The two major monocyte subsets in humans, mouse, and rat. The classical and non-classical subsets differ with regard to size, granularity and the expression of surface markers. There is an intermediate subset of monocytes that was recently described which is not included in this diagram.
|
|||||||||||
|
| |||||||||||||