Transgenerational Regulation
Changes that persist for many generations without altering genome sequence currently lack good explanations. For any such heritable epigenetic change, an adequate explanation needs to answer three questions: 1) How many generations does it last? 2) What molecules are required? 3) What change in regulatory architecture accounts for its nature and duration?
Phenomena like paramutation and RNA interference (RNAi) have revealed that changes in gene regulation can persist for many generations. Such transgenerational gene silencing can be initiated in C. elegans using double-stranded RNA (dsRNA) or a class of small RNAs called piRNAs. We recently discovered another phenomenon whereby mating can reliably initiate transgenerational gene silencing. These different ways of initiating transgenerational gene silencing provide opportunities to understand how molecules that can change the heritable information gain access to the germline and how induced changes are transmitted across generations.
Initiation by the entry of double-stranded RNA into the germline: The somatic cells that generate our body are separated during early development from the germ cells that make sperm or egg. Therefore, any molecules that can change gene regulatory information need to gain access to the germline to cause transgenerational effects. Early experiments using the injection of dsRNA or feeding worms bacteria that express dsRNA suggested that dsRNA and/or molecules derived from it could be transported to the germline in C. elegans.
Injection: To directly visualize the fate of extracellular dsRNAs, we injected fluorescently labeled 50-bp dsRNA into the body cavity that surrounds all tissues, including the germline, in C. elegans. We found that dsRNA entered oocytes along with yolk and reached progeny (Marré et al., 2016). Surprisingly, we found that such delivery of extracellular RNA from parent to progeny did not require entry into the cytosol within the parent. Entry into the cytosol can subsequently occur in progeny through the dsRNA importer SID-1 - a conserved protein with homologs in many animals, including humans. 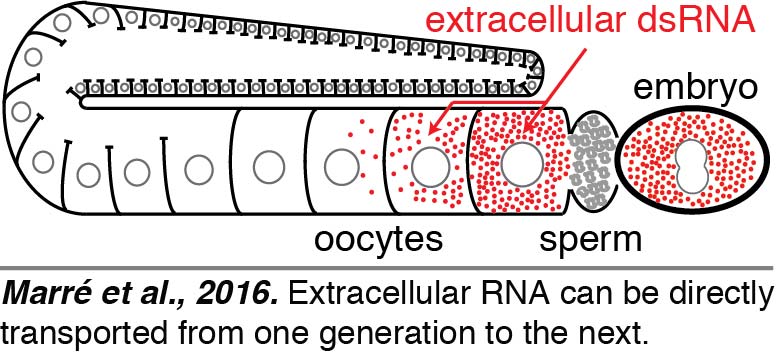 Thus, the dsRNA that enters oocytes along with yolk can presumably be held within intracellular vesicles and reach embryos. These results raise the intriguing possibility that extracellular RNAs that are secreted – potentially in response to changes in a parent – can directly reach progeny and regulate gene expression. However, other studies suggest additional routes for the transport of dsRNA into germ cells that remain to be elucidated.
Thus, the dsRNA that enters oocytes along with yolk can presumably be held within intracellular vesicles and reach embryos. These results raise the intriguing possibility that extracellular RNAs that are secreted – potentially in response to changes in a parent – can directly reach progeny and regulate gene expression. However, other studies suggest additional routes for the transport of dsRNA into germ cells that remain to be elucidated.
Feeding: A robust and easy way to initiate RNA silencing is feeding worms bacteria that express dsRNA (feeding RNAi). 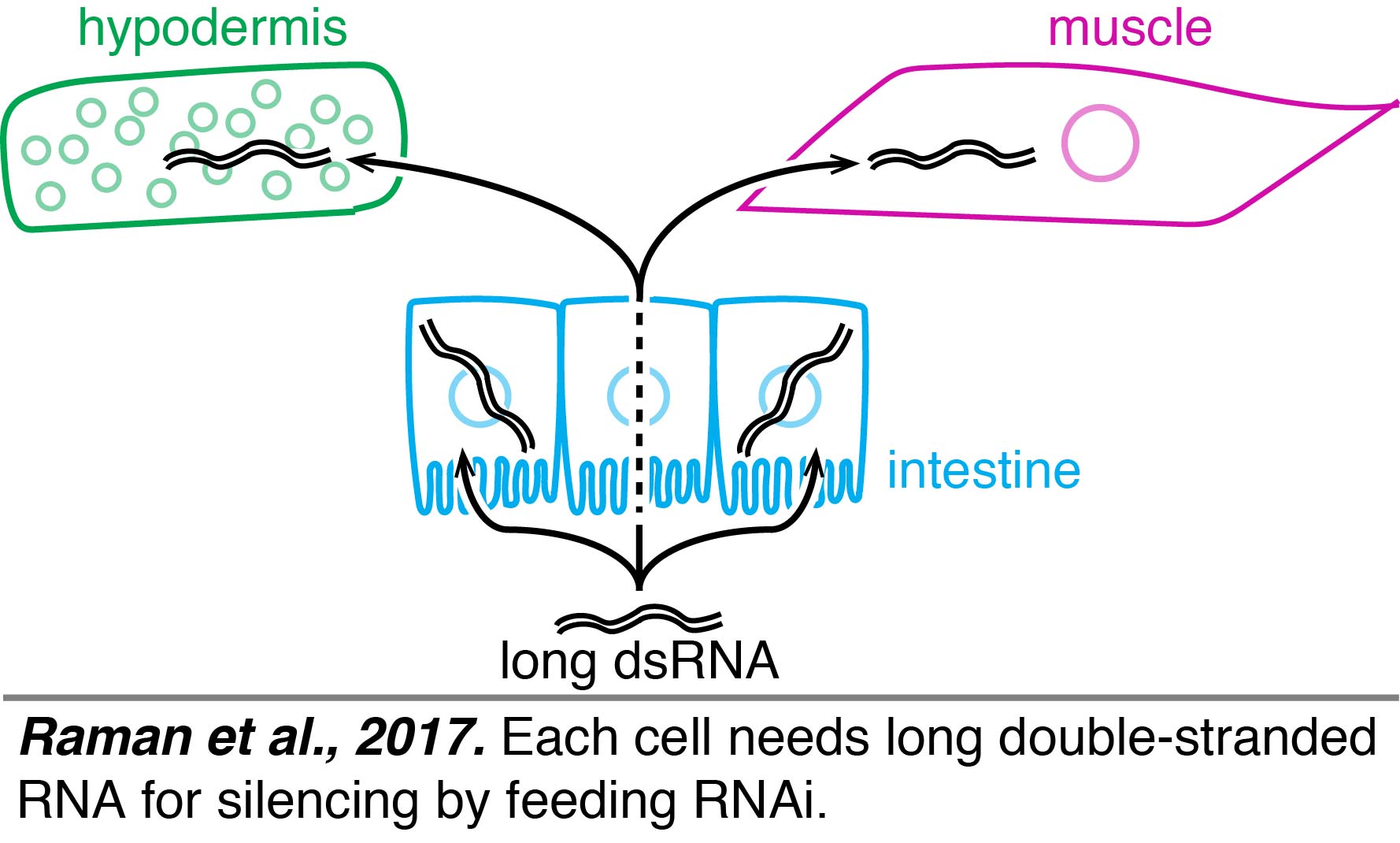 Using feeding RNAi, multiple groups have contributed to an understanding of how long dsRNA is processed into shorter dsRNAs and used for finding matching sequences to cause gene silencing. One way to infer what RNAs need to enter each tissue to cause silencing is by restricting the processing of long dsRNA to specific tissues. Such tissue-specific rescue experiments using single-copy transgenes suggest that each cell needs long dsRNA for silencing by feeding RNAi (Raman et al., 2017).
Expression: Recent work in many organisms is revealing that cells can communicate with each other through the direct transport of macromolecules (e.g. RNA). Secreted vesicles with such macromolecules that are found in human blood are being hotly pursued as valuable diagnostic markers for the health of internal organs. However, the mechanisms that enable this mode of cell communication are not well understood.
We found that dsRNA expressed within neurons could enter the germline and cause transgenerational gene silencing (Devanapally et al., 2015).
Using feeding RNAi, multiple groups have contributed to an understanding of how long dsRNA is processed into shorter dsRNAs and used for finding matching sequences to cause gene silencing. One way to infer what RNAs need to enter each tissue to cause silencing is by restricting the processing of long dsRNA to specific tissues. Such tissue-specific rescue experiments using single-copy transgenes suggest that each cell needs long dsRNA for silencing by feeding RNAi (Raman et al., 2017).
Expression: Recent work in many organisms is revealing that cells can communicate with each other through the direct transport of macromolecules (e.g. RNA). Secreted vesicles with such macromolecules that are found in human blood are being hotly pursued as valuable diagnostic markers for the health of internal organs. However, the mechanisms that enable this mode of cell communication are not well understood.
We found that dsRNA expressed within neurons could enter the germline and cause transgenerational gene silencing (Devanapally et al., 2015). 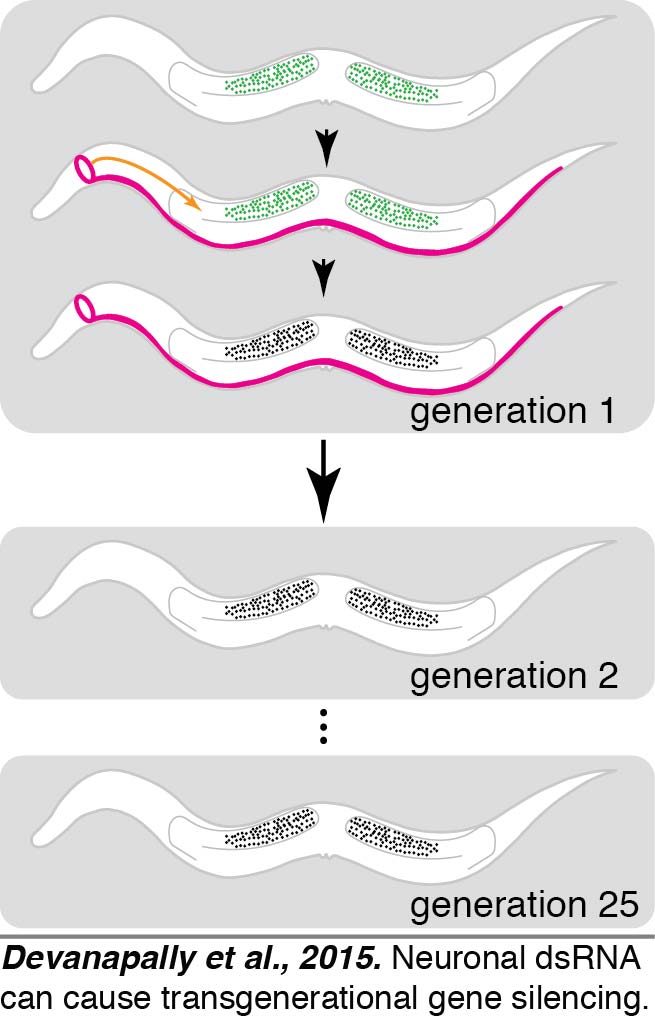 C. elegans neurons (magenta) can send gene-specific messages in the form of dsRNA to the germline (green) and cause transgenerational gene silencing that can last for more than 25 generations. Neuronal dsRNAs enter germ cells through SID-1. These results can have profound implications for our understanding of evolution and behavior. A UMD-funded program called the Transgenerational Brain Initiative that trained ~35 undergraduates each year in original research aimed at discovering which neuron(s) are the best exporters of dsRNA to the germline. Current models for the transgenerational inheritance of silencing in C. elegans propose reinforcement through the production of small RNAs and associated chromatin modifications in each generation. Most aspects of this model, however, remain to be tested and only a few protein factors required for this process are known.
C. elegans neurons (magenta) can send gene-specific messages in the form of dsRNA to the germline (green) and cause transgenerational gene silencing that can last for more than 25 generations. Neuronal dsRNAs enter germ cells through SID-1. These results can have profound implications for our understanding of evolution and behavior. A UMD-funded program called the Transgenerational Brain Initiative that trained ~35 undergraduates each year in original research aimed at discovering which neuron(s) are the best exporters of dsRNA to the germline. Current models for the transgenerational inheritance of silencing in C. elegans propose reinforcement through the production of small RNAs and associated chromatin modifications in each generation. Most aspects of this model, however, remain to be tested and only a few protein factors required for this process are known.
Despite the progress in understanding how extracellular dsRNA causes silencing within the germline, many questions remain: (1) How is dsRNA exported from cells? (2) What is the full complement of routes taken by dsRNA from one generation to the next? (3) What RNAs transcribed from the C. elegans genome or from ingested bacteria reach the germline? (4) What other gene regulatory molecules are transported across generations?
Initiation by mating: The recent ability to precisely modify the C. elegans genome led several groups 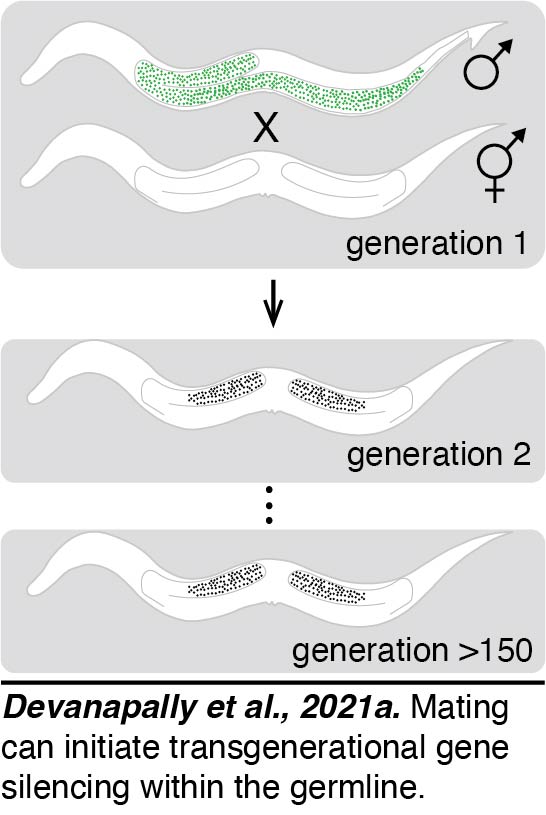 to uncover a transgenerational gene silencing response that can act on single-copy transgenes. This silencing resembles paramutation and was shown to depend on a class of small RNAs called piRNAs. We found a single-copy transgene that shows stable expression but that can be silenced simply by mating males with the transgene to hermaphrodites that lack the transgene - a phenomenon we named mating-induced silencing (Devanapally et al., 2021a). This silencing can be stable for >300 generations. Genetic requirements for this phenomenon suggest that initiation of silencing requires piRNAs and maintenance of silencing requires the production of additional small RNAs. Because mating is an easy approach to reliably initiate transgenerational silencing, it can be coupled with CRISPR-based genetic and epigenetic engineering approaches to uncover the features that determine transgenerationally stable expression versus silencing.
Our future efforts will address the following questions:
(1) What features of a transgenic locus determine susceptibility to mating-induced silencing?
(2) Are there endogenous loci that are susceptible to mating-induced silencing?
(3) What regulatory mechanisms determine whether a gene is expressed or silenced?
(4) How can such mechanisms be changed to reconfigure expression of a gene for many generations?
to uncover a transgenerational gene silencing response that can act on single-copy transgenes. This silencing resembles paramutation and was shown to depend on a class of small RNAs called piRNAs. We found a single-copy transgene that shows stable expression but that can be silenced simply by mating males with the transgene to hermaphrodites that lack the transgene - a phenomenon we named mating-induced silencing (Devanapally et al., 2021a). This silencing can be stable for >300 generations. Genetic requirements for this phenomenon suggest that initiation of silencing requires piRNAs and maintenance of silencing requires the production of additional small RNAs. Because mating is an easy approach to reliably initiate transgenerational silencing, it can be coupled with CRISPR-based genetic and epigenetic engineering approaches to uncover the features that determine transgenerationally stable expression versus silencing.
Our future efforts will address the following questions:
(1) What features of a transgenic locus determine susceptibility to mating-induced silencing?
(2) Are there endogenous loci that are susceptible to mating-induced silencing?
(3) What regulatory mechanisms determine whether a gene is expressed or silenced?
(4) How can such mechanisms be changed to reconfigure expression of a gene for many generations?
Transgenerational homeostasis in the germline: We discovered that genes can generally recover from transgenerational RNA silencing within the germline(Devanapally et al., 2021b). This recovery likely includes 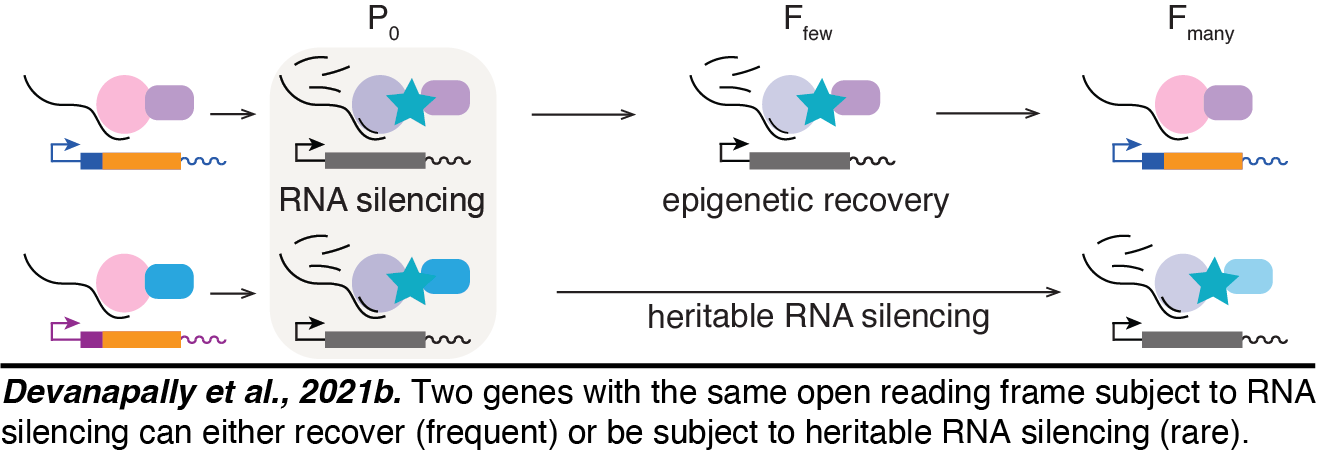 active mechanisms that oppose or repair epigenetic change within the germline. Such 'epigenetic repair' could protect the epigenome just as DNA repair protects the genome. Yet, we have identified rare gene contexts that can make sequences vulnerable to transgenerational epigenetic inheritance such that once initiated RNA silencing can stably persist for more than 300 generations. One possibility is that such change occurs because homeostatic mechanisms that operate across generations are not engaged in these rare gene contexts. Additional studies are needed to explore this possibility and other explanations.
active mechanisms that oppose or repair epigenetic change within the germline. Such 'epigenetic repair' could protect the epigenome just as DNA repair protects the genome. Yet, we have identified rare gene contexts that can make sequences vulnerable to transgenerational epigenetic inheritance such that once initiated RNA silencing can stably persist for more than 300 generations. One possibility is that such change occurs because homeostatic mechanisms that operate across generations are not engaged in these rare gene contexts. Additional studies are needed to explore this possibility and other explanations.
Recovery from RNA interference in somatic cells: Inspired by our exploration of a quantitative model for RNA interference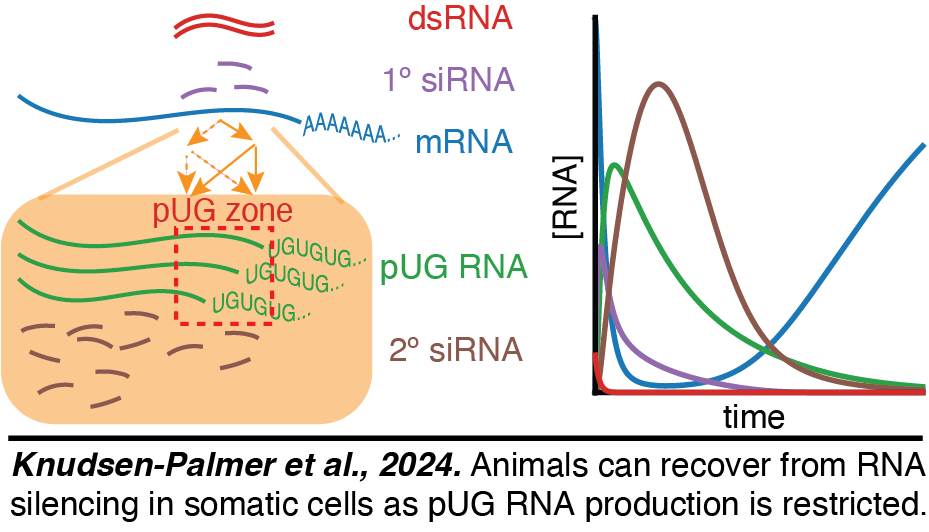 , we discovered that animals can recover from a pulse of RNA interference even when the double-stranded RNA (dsRNA) targets mRNA within non-dividing somatic cells. This recovery implies that all intermediates of RNA silencing (22G RNA, pUG RNA, etc.) undergo turnover and that there is limited amplification of small RNAs (Knudsen-Palmer et al., 2024). Using an RT-PCR based assay we found that pUG RNA production is most abundant in the region of the target mRNA that matches the sequence of the added dsRNA. This restriction likely limits amplification to one round of 22G RNA production, explaining the eventual recovery from RNA silencing.
, we discovered that animals can recover from a pulse of RNA interference even when the double-stranded RNA (dsRNA) targets mRNA within non-dividing somatic cells. This recovery implies that all intermediates of RNA silencing (22G RNA, pUG RNA, etc.) undergo turnover and that there is limited amplification of small RNAs (Knudsen-Palmer et al., 2024). Using an RT-PCR based assay we found that pUG RNA production is most abundant in the region of the target mRNA that matches the sequence of the added dsRNA. This restriction likely limits amplification to one round of 22G RNA production, explaining the eventual recovery from RNA silencing.
Buffering heritable epigenetic changes: We found that when the import of dsRNA into the germline is prevented by eliminating SID-1, heritable RNA silencing within the germline is enhanced. The loss of SID-1 alters 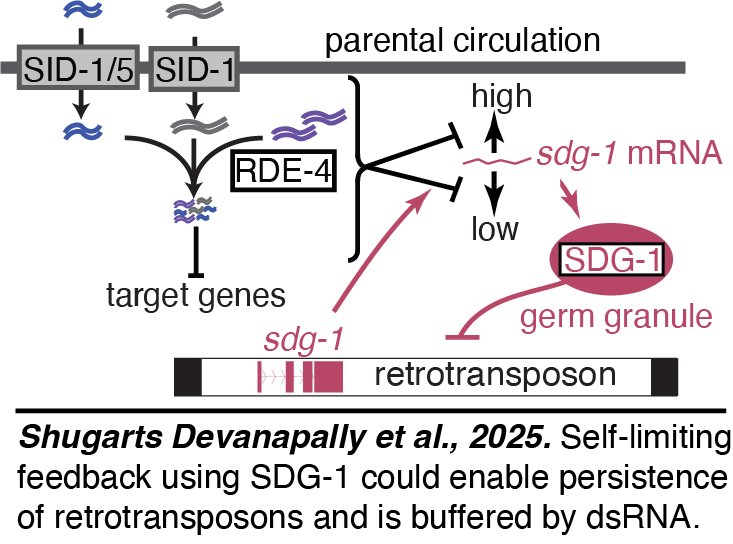 the expression of multiple genes within the germline and these changes can persist for more than 100 generations even after wild-type SID-1 is restored. The first of these sid-1-dependent genes, sdg-1, is located within a retrotransposon and yet encodes a protein that localizes to perinuclear germ granules that are required for the silencing of retrotransposons. Together, these discoveries suggest that the entry of extracellular dsRNA into the germline buffers heritable epigenetic changes and that the SDG-1-mediated autoregulatory loop limits the silencing of retrotransposon, providing such 'selfish elements' a strategy for persistance (Shugarts Devanapally et al., 2025).
the expression of multiple genes within the germline and these changes can persist for more than 100 generations even after wild-type SID-1 is restored. The first of these sid-1-dependent genes, sdg-1, is located within a retrotransposon and yet encodes a protein that localizes to perinuclear germ granules that are required for the silencing of retrotransposons. Together, these discoveries suggest that the entry of extracellular dsRNA into the germline buffers heritable epigenetic changes and that the SDG-1-mediated autoregulatory loop limits the silencing of retrotransposon, providing such 'selfish elements' a strategy for persistance (Shugarts Devanapally et al., 2025).
Multiple forms of heritable RNA silencing: 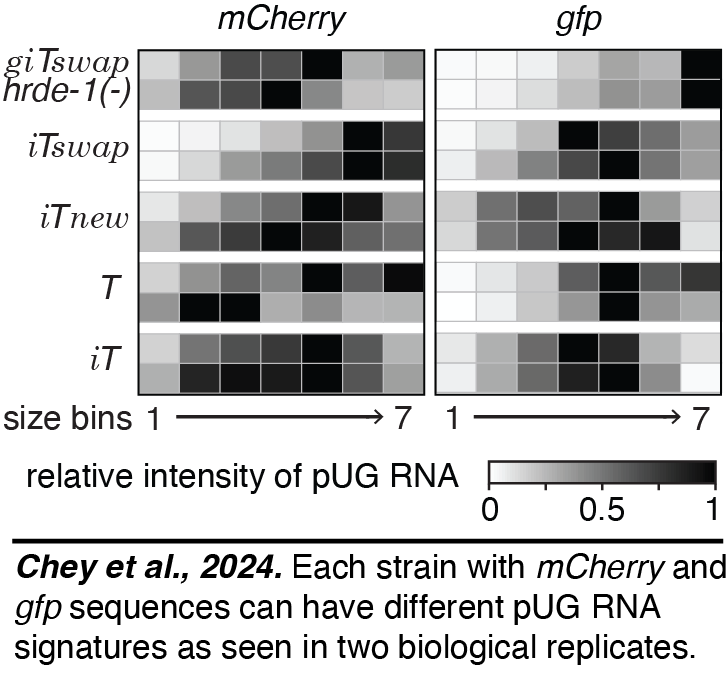 Using two-gene operons, we provided evidence for multiple heritable epigenetic states for the same open reading frame. Some states are characterized by different genetic requirements (requiring or not requiring the Argonaute protein HRDE-1). Some are associated with distinct patterns of pUG RNAs (Chey et al., 2024). These various epigenetic states that are each stable for many generations can be considered different 'epialleles' of the same gene. We expect that the pUG RNA signatures that characterize each state should enable epigenetic analyses just like different mutants of a gene enable genetic analyses.
Using two-gene operons, we provided evidence for multiple heritable epigenetic states for the same open reading frame. Some states are characterized by different genetic requirements (requiring or not requiring the Argonaute protein HRDE-1). Some are associated with distinct patterns of pUG RNAs (Chey et al., 2024). These various epigenetic states that are each stable for many generations can be considered different 'epialleles' of the same gene. We expect that the pUG RNA signatures that characterize each state should enable epigenetic analyses just like different mutants of a gene enable genetic analyses.
Back to research
Last updated: Dec 2025