Pharmacology of the Autonomic
Nervous System:
Utilizing the Isolated Ileum Model System
I. Introduction
Mammalian smooth muscle
typically occurs as sheets of contractile fibers
surrounding the intestinal tract, uterus, spleen
and blood vessels. It is both anatomically
and physiologically different from skeletal muscle.
The fibers are short, narrow, spindle-shaped cells
with little sarcoplasmic reticulum. The highly
ordered structure of skeletal muscle is absent,
and the contractile properties reflect the differences
in structure. Smooth muscle contraction is
slow, sustained, and spontaneous. If smooth
muscle tissue is stretched, it will first relax
to this new length, and then begin to resume spontaneous
activity. Therefore, the muscle can work over
a wide range of lengths, a definite advantage for
a hollow organ (e.g., intestine, uterus or blood
vessel) subject to distention due to filling.
Smooth muscle contractions
occur in the absence of any innervation (unlike
skeletal muscle contractions). However, smooth
muscle activity is mediated by the two divisions
of the autonomic nervous system, sympathetic and
parasympathetic. The sympathetic innervation
is inhibitory, while the parasympathetic is excitatory.
In the intestinal smooth muscle preparation utilized
in these experiments, the parasympathetic ganglia
and the post synaptic receptors are contained in
the tissue. However, the sympathetic ganglia
are absent. Why is this?
Review the structure and pharmacology of the
autonomic nervous system to familiarize yourself
with these details.
II. Summary of Experimental Procedures
You will isolate a segment
of rat ileum and suspend it in an isotonic buffered
saline solution (Tyrode's). One end of the
muscle is fixed while the other end is attached
to a force-displacement transducer for recording
muscle contractions. You will use this preparation
to demonstrate and examine:
1. The principles
of a bioassay and Dose-Response curves;
2. The effects of
sympathetic and parasympathetic agonists and antagonists;
3. The use of this
preparation in order to determine the site of action
of
an unknown agent.
All of the data will be recorded on disk
using the WINDAQ/200 program and then analyzed
using the WINDAQ playback feature (see
the sections in this manual on the use of these
programs).
III. METHODS
Data Recording: While one members
of the group prepares the tissue, another member
can set up the Transbridge with a FORT-10, force-displacement
transducer and ready the WINDAQ program for recording
on one channel. Since you will be examining
contractions that invoke an upward deflection of
the computer screen trace, set the baseline (0 tension)
near the bottom of the screen. The resulting
changes in muscle tension are slow to occur but
last up to 4 or 5 minutes. Select a slow sampling
rate, e.g., 10-50/ sec.
Do not tweak, flick, or bend the transducer!!!
It is a fragile instrument!
Dissection Techniques: Your TA will
mercifully dispatch the rat with CO2 narcosis.
After the animal stops breathing, make an abdominal
midline incision to expose the viscera. Take
care to avoid cutting any visceral organs.
Expose the viscera and cut the diaphragm at the
top of the abdominal cavity.
Note any spontaneous movements
of the intestine in situ. Carefully
lift out the intestine without stretching
it and lay it in a Petri dish containing Tyrode's
solution warmed to room temperature. Locate
a segment of the small intestine as close to the
ileo-cecal junction as possible and cut away the
mesentery beginning at this point and proceeding
for 10 - 15 cm toward the stomach. Handle
the intestine gently and keep it moist. (Feel free
to perform a dissection of the rat. Your TA will
point out the major organs.)
Sever the ileum as close
to the colon as possible and again about 12 -15
cm toward the mouth. Place this isolated section
into a separate Petri dish containing fresh Tyrode's
solution. Flush and rinse out the lumen by gently
forcing Tyrode's through it with a 5 ml syringe.
Place this rinsed section of intestine into another
Petri dish containing fresh Tyrode's solution.
Do not let it touch or sit in fluid containing
the intestinal contents.
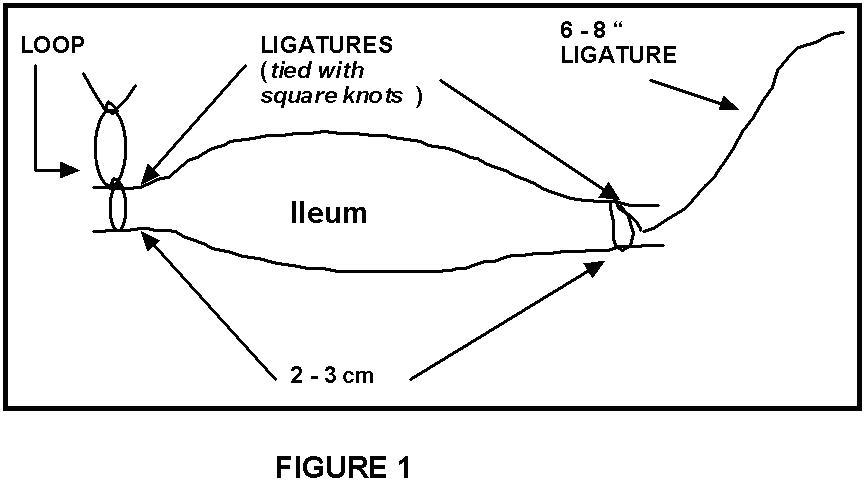
Attach surgical thread
to one end of the ileum. One end of this thread
should be left about 10-12 inches long (see Figs
1 & 2). Be sure to tie square knots;
no granny knots, please! A second thread should
be tied about 2.0 - 3.0 cm down the ileum from the
first thread. The ends of this second thread
should be formed into a small loop with another
square knot (Figs. 1 & 2). Carefully
isolate this segment of ileum by cutting it outside
of the attached ligatures. Repeat this procedure
on the remaining length of ileum until one segment
has been prepared for each lab group. Figure
1 illustrates a resulting section of ileum.
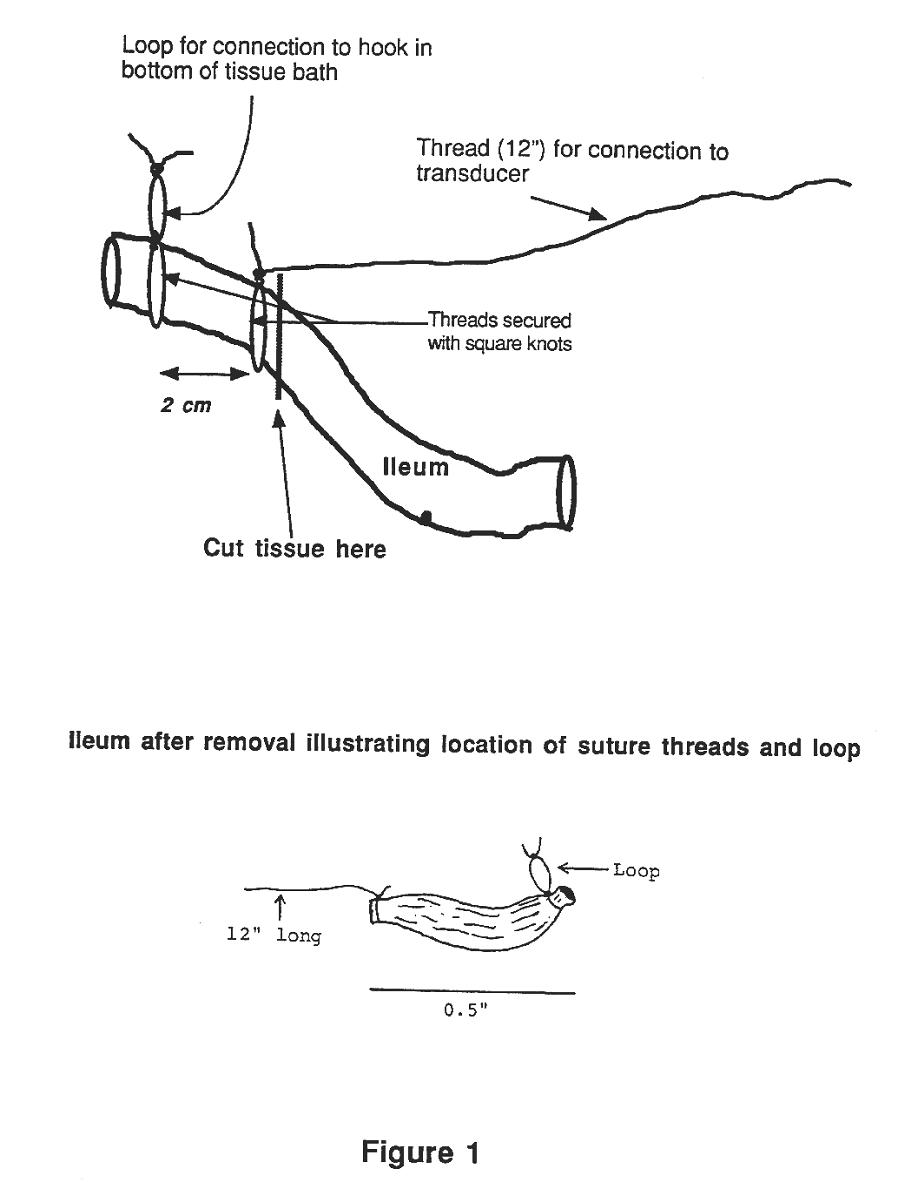
Attach one end of your
segment to the hook in the bottom of the tissue
bath ( see Fig. 2 & 3) using the small
loop in the thread. Attach the long thread
attached to the other end of the muscle to the lever
of the transducer. Tie the ileum FIRST!!!!
Then, move the transducer up in order to
place a very small amount of tension on the ileum
segment. Watch the screen in order to determine
when you start to place tension on the transducer.
Be careful not to damage the transducer!!!
Fill the bath with a Tyrode's solution by clamping
the tubing at the base of the tissue bath and adding
Tyrode’s to the top.
Check the setup to make
certain that the suture is as vertical as possible
and is not touching the side of the tissue bath
and the muscle has a slight tension on it.
Very gently, start aerating the solution using the
attached air pump and record the contractions using
the WINDAQ data acquisition program. The sampling
rate should be set at a low frequency. After
suspending the muscle but before adding any drugs,
calibrate the transducer with a 10 g weight.
You will probably also need to turn the gain down
on the transbridge amplifier, probably to x100 or
even x10 in order to observe full contraction of
the ileum segment.
IV. EXPERIMENTS
A. Bioassay for Acetylcholine (ACh)
ACh interacts with post
synaptic cholinergic receptors on the ileum to initiate
and potentiate contractions. Remember that
the most peripheral receptors are affected first.
(So, are these nicotinic or muscarinic?) The
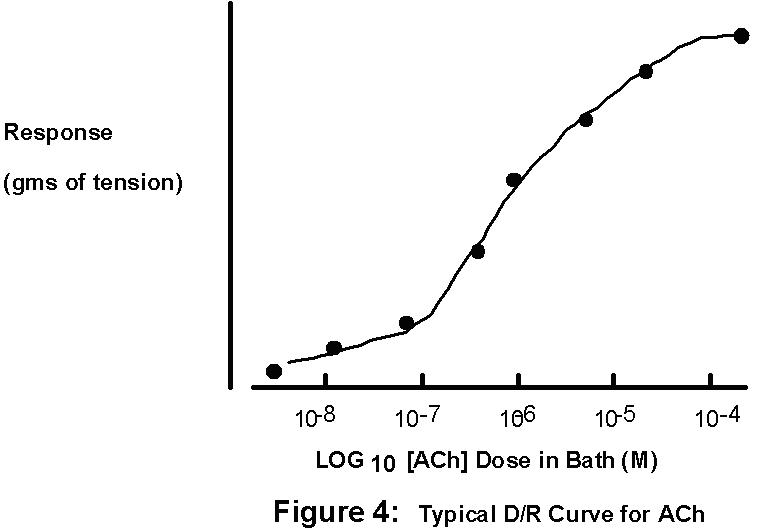
magnitude of the resulting contraction is proportional
to the dose of ACh applied within a restricted range
of ACh concentrations which varies from muscle to
muscle. You will take advantage of this to
construct a Dose-Response curve and then use this
standard curve (see Bsci 105 and Bsci 230 lab manuals!)
to determine the [ACh] of an "unknown" solution.
Stock ACh solution will
be available at a concentration of 1 x 10-1
M (100 mM). Make a serial dilution of this
solution by placing 0.5 ml of the stock ACh in 4.5
ml of Tyrode's solution; this is equivalent to a
10-fold dilution of the stock ACh that yields a
10-2 M ACh solution. Take a 0.5
ml of this 10-2 M solution and add 4.5
ml of Tyrode and you will now have a 10-3
M solution of ACh. Continue this until you
have ACh solutions ranging from 10-2
M to 10-7 M. Note that these dilutions
provide a range over which you can test the ileum's
response. You can make other ACh dilutions within
a more narrow range once you have determined how
your particular ileum responds. You will want to
use at least 8 different [Ach] in order to get a
complete picture of your dose-response curve. Where
should these 8 [Ach] lie along the x-axis? That
is which concentrations will provide the most information
about the shape of your dose-response curve?
Do you understand log doses and dilutions???
To be sure, complete the following calculations
before going any further. Remember that you can
use the formula C1 x V1 = C2 x V2, (C = concentration,
V= volume). The trick is in determining how to assign
the variables. Your TA will elaborate.
1. If you dilute 1.0 ml
of a 10-5M ACh solution with 1.0 ml of
Tyrode's buffer, the [ACh] now = __________________________________.
2. After adding 0.2 ml
of 10-4M ACh to 20 ml of Tyrode's buffer,
the [ACh] now =
_____________________________________.
3. After adding another
0.2 ml of 10-4M ACh to the same 20 ml
of Tyrode's buffer in #2 above, the [ACh] now =
_____________________________________.
4. If you add 0.4 ml of
an ACh stock solution to 20 ml of Tyrode's and found
that you now have a final [ACh] of 2 x 10-6M
in Tyrode's, what was the [ACh] in the original
stock solution? ____________________________________________.
But, we haven't yet considered the fact that the
dose of Ach gets "diluted" when it is added to the
volume of liquid in the tissue bath. For example
if you add the following aliquots, what is the final
[ACh] in the bath if the initial bath volume
is 20 ml?
0.2 ml of 10-6
M ACh giving a [ACh]bath = ___________M
0.2 ml of 10-5
M ACh giving a [ACh]bath = ___________M
0.2 ml of 10-4
M ACh giving a [ACh]bath = ___________M
0.2 ml of 10-3
M ACh giving a [ACh]bath = ___________M
After constructing your protocol (stock doses,
volumes to be added, and resulting bath concentrations):
1. Start recording the
data in the computer and quickly add the first sample
(lowest dose) of ACh, waiting approximately 1 min.
for a response. Wait approximately 1 full min before
assuming no response. Once you witness a contraction,
you have a very rough estimate of the log unit threshold
dose of ACh for this section of this particular
ileum. Plan your protocol of ACh additions
to include several (>8) doses of ACh that are
between threshold and maximum.
2. Add the next volume
increment, without flushing the bath.
3. Once a contraction is
evoked, the timing of your next addition becomes
critical. As soon as this initial contraction reaches
a maximum and the trace begins to plateau, add the
next dose. A series of doses can then be added to
the muscle, without flushing out the organ bath
between each addition. This method is based on the
relatively slow response and recovery time of the
smooth muscle. By the time the muscle has reached
maximal contraction, the added ACh has essentially
equilibrated among the accessible tissue compartments.
At this point, the muscle is in a state of contraction
proportional to the concentration of exogenous ACh.
Therefore, if the next dose of ACh is then added
(at the plateau point of the proceeding dose), the
muscle will contract to a length proportional to
the total dose now in the bath. How can you
determine the TOTAL [ACh] in the bath at each step?
(HINT: must consider total AMOUNT of ACh added and
then calculate the total volume of liquid in the
bath at each step.)
In order to determine the final [ACh] in the tissue
bath, you will have to add the doses of ACh
(in moles), beginning with the most dilute solution,
as well as consider the incremental increase in
bath volume with each added 0.2 ml dose.
4. Now graph your results
on semi-log graph paper. Simply place your doses
of ACh on the X axis (the logarithmic axis) and
the corresponding muscle responses (in grams of
tension) on the Y axis (linear axis). Why is it
often necessary to plot the dose on a logarithmic
scale?
B. *ACh Antagonist*
1. Obtain a representative
ACh dose-response (D/R) curve.
2. Duplicate the curve
in the presence of 10-7 M (bath dose!)
atropine, a
muscarinic antagonist. Plot the
curves obtained in A & B on the same axes.
3. Predict what will happen
if the dose of the antagonist was increased or decreased.
4. Predict what will happen
if the ACh D/R curve were generated in the presence
of
hexamethonium. (After C, try it!!!!)
C. Inhibition of Acetylcholinesterase
(AChase)
AChesterase is an enzyme
located on cholinergic post synaptic membranes.
AChesterase hydrolyzes ACh into choline and acetate
which do not interact with the ACh receptors.
Demonstrate the effect of an AChase inhibitor, eserine
(10-6 - 10-7 M), on the ileum's
response to ACh. Report your results as dose-response
curves for A, B & C plotted on the same axes.
D. (WEEK 2) Determining the Site of Action
of an Unknown Agent
You will be given a solution
containing an unknown agent. You must use the ileum
preparation to determine its site of action.
The agent may be any one of the following:
• muscarinic
agonist or antagonist
• nicotinic
agonist or antagonist
• alpha
adrenergic agonist or antagonist
• beta
adrenergic agonist or antagonist
• nonspecific
muscle contraction or relaxing agent
Before you arrive in lab for week 2, construct
a flow chart of all possible results induced by
an unknown agent. Thus, the drug may elicit contraction,
relaxation, or no response. If it induces
contraction of the ileum, what are the possibilities?
It could be a muscarinic agonist or a nicotinic
agonist. What drugs or sequence of drugs would you
apply to distinguish among these possibilities?
Consider this scenario: if the unknown was blocked
by atropine but not by hexamethonium, what would
your conclusion be? What if it weren't blocked by
either?
Now construct a flow chart for determining the identity
of an unknown agent that produces ileum relaxation….no
response…
Suggested Strategy:
1. Test muscle with ACh to determine tissue viability.
Wash thoroughly.
2. Apply unknown.
3. Employ your flow chart of possible results and
the isolated ileum preparation to actually determine
the site of action of an unknown drug. Note that
you may need to apply a small dose of Ach in
order to generate some tension before you are actually
able to see any relaxation elicited by an adrenergic
agonist.
Back to the top